��Ŀ����
ij��ѧʵ��С��ʵ�����ʱ��������CuSO4��ZnSO4��FeSO4�ķ�Һ���ڷ�Һ���Ϊ�����йؽ������Σ�ͬѧ�����������ʵ�鷽����
 �Իش�
�Իش�
(1)�������п�۱��������ԭ����______________________
(2)д�����������һ��Ӧ�Ļ�ѧ����ʽ__________________
(3)���鲽����м����ϡ�����Ƿ������ķ�����_______
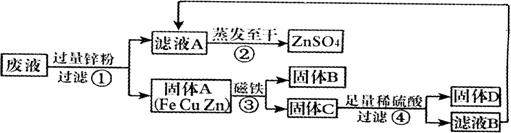
(4)ָ����ͼ�Ĺ��˲����Ĵ��� ��
(5)��ʵ������е�������ʧ���ԣ�������������п������
������ڡ�����С�ڡ����ڡ���ԭ��Һ������п��������Ҫ����÷�Һ������ͭ����������Ҫ���� ��������
ÿ��1�֣��ڣ�4�����ȫ��1�֣����÷�
��1���û�����Һ�����е�ͭ������2��Zn+CuSO4==Cu+ZnSO4(Zn+FeSO4==Fe+ZnSO4)��3����D�м���ϡ���ᣬ������������������4��������������
��4��δ�ò����������ձ��е�Һ�壬��ֽ��Ե����©����Ե
��5�����ڣ�__����D______

��ϰ��ϵ�д�
�����Ŀ
 ̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
ʵ��һ��̽��̼��������Һ�������
ȡ̼��������Һ�������μ���ɫ��̪��Һ��죬�ɴ˿�֪̼��������Һ��______�ԣ�
ʵ�����̽��̼�����Ƶ����ȶ���
[��������]̼�������������ֽ⣬����ˮ��������̼�����һ�ֳ����Ĺ������ʣ�
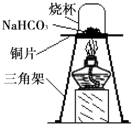
[����ʵ��]Ϊ��֤̼����������ʱ��ֽ⣬��ȤС���ͬѧȡһ��������̼�����Ƶ�ͭƬ�ϼ��ȣ���ͼ��ʾ��
��1������һ��ʱ��۲쵽�ձ��ڱ���______���ɣ�
��2����ּ��Ⱥ��ձ�Ѹ�ٵ�ת���������������ij���ʯ��ˮ�����۲쵽ʯ��ˮ����ǣ�д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����ȤС���ͬѧ��Ϊ��ּ��Ⱥ�Ĺ�����������NaOH��Na2CO3��
����ȤС���ͬѧ�����������______��
�������ʵ����鷴Ӧ��Ĺ��������NaOH��Na2CO3���������±���
| ʵ���顡�١��� | Ԥ��ʵ������ | �ᡡ���� |
| ȡ���������������м���______�� | �����ݲ��� | ���������Na2CO3��������NaOH�� |
 ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����
ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽���� ��ѧʵ���г�����������������������������ѧ�����������û��ᣬҪ���ݾ����������Դ������ڷ��ֺͽ�����⣮���磺
��ѧʵ���г�����������������������������ѧ�����������û��ᣬҪ���ݾ����������Դ������ڷ��ֺͽ�����⣮���磺