��Ŀ����
����ӵ�зḻ��������Դ�����ǿ��ԴӺ�ˮ����ȡʳ�Σ����Դ�Ϊԭ���Ƶþ��й㷺��;���ռ�Ȼ�����Ʒ������ȡ����Ҫ�����������£�

��1�����÷紵��ɹ���ԴӺ�ˮ����ȡ���Σ��紵��ɹ����Ҫ������
��2��ʵ���ҽ��д��εij����ᴿʱ��һ��Ҫ�����ܽ⡢
��3��д���Ȼ��Ʊ�����Һ��ͨ�������·�����Ӧ�Ļ�ѧ����ʽ

��1�����÷紵��ɹ���ԴӺ�ˮ����ȡ���Σ��紵��ɹ����Ҫ������
�ٽ�ˮ������
�ٽ�ˮ������
����2��ʵ���ҽ��д��εij����ᴿʱ��һ��Ҫ�����ܽ⡢
����
����
�������ᾧ�������裮�������ᾧ�����У�Ҫʹ�õ���Ҫ�����д���Ȧ������̨���ƾ��ơ���������������
������
�����в����������������裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ�
���裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ�
����3��д���Ȼ��Ʊ�����Һ��ͨ�������·�����Ӧ�Ļ�ѧ����ʽ
2NaCl+2H2O
2NaOH+H2��+Cl2��
| ||
2NaCl+2H2O
2NaOH+H2��+Cl2��
��
| ||
��������1�����ݷ���Խ���¶�Խ�ߣ�ˮ������Խ����н��
��2�����ݴ����ᴿ�IJ����Լ������������ý��н��
��3�����ݵ�ⱥ��ʳ��ˮʱ���������������ơ��������������н��
��2�����ݴ����ᴿ�IJ����Լ������������ý��н��
��3�����ݵ�ⱥ��ʳ��ˮʱ���������������ơ��������������н��
����⣺��1���紵��ɹ����Ҫ�����Ǵٽ�ˮ������������ٽ�ˮ��������
��2��ʵ���ҽ��д��εij����ᴿʱ��һ��Ҫ�����ܽ⡢���˺������ᾧ�������裮�������ᾧ�����У�Ҫʹ�õ���Ҫ�����д���Ȧ������̨���ƾ��ơ������������������в������������ǽ��裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ���������ˣ��������裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ���
��3���Ȼ��Ʊ�����Һ��ͨ�������·�����Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2�������2NaCl+2H2O
2NaOH+H2��+Cl2����
��2��ʵ���ҽ��д��εij����ᴿʱ��һ��Ҫ�����ܽ⡢���˺������ᾧ�������裮�������ᾧ�����У�Ҫʹ�õ���Ҫ�����д���Ȧ������̨���ƾ��ơ������������������в������������ǽ��裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ���������ˣ��������裬ʹҺ�����Ⱦ��ȣ���ֹ�ɽ���
��3���Ȼ��Ʊ�����Һ��ͨ�������·�����Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O
| ||
| ||
������������Ҫ�����������ܽ⡢��ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


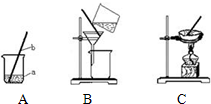 ��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������
��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������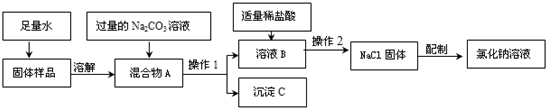
 ��2012?���壩ϡ��Ԫ���ڿƼ��������й㷺����;������Ϊ�²��ϵı��⣮�ҹ�ӵ�зḻ��ϡ����Դ��Լռ����ϡ����Դ��80%���ң���ͼΪϡ��Ԫ������Ԫ�����ڱ��е���Ϣ������˵����ȷ���ǣ�������
��2012?���壩ϡ��Ԫ���ڿƼ��������й㷺����;������Ϊ�²��ϵı��⣮�ҹ�ӵ�зḻ��ϡ����Դ��Լռ����ϡ����Դ��80%���ң���ͼΪϡ��Ԫ������Ԫ�����ڱ��е���Ϣ������˵����ȷ���ǣ�������