��Ŀ����
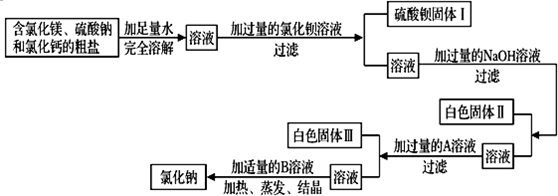
�����Ƿḻ�Ļ�ѧ��Դ���⣮ͨ����ɹ��ˮ���Եõ����н϶����ʣ����ʰ���CaCl2��MgSO4����ɳ�ȣ��Ĵ��Σ�
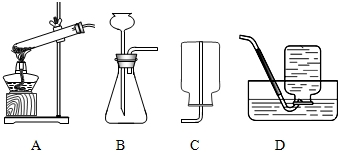
 ��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������
��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������
�ٲ���C�п���
�ھ������������������ܷ�õ�������ʳ��
��2��ij������Ʒ20�ˣ����п����Ե��Ȼ������ʺͲ����Ե���ɳ�������ʺ��Բ��ƣ��ֳɶ��ȷݣ�
�ٳ����£���һ�ݸô�����Ʒ��ȫ�ܽ���һ����ˮ�У����˵õ�1.5����ɳ��
��ѧУ��ѧ��ȤС�齫��һ�ݴ�����Ʒ������һ������������NaCl��Һ��ʵ���������ͼ��������ÿһ����Ʒ����ɳ���ȵ����ķֲ���
��ش�
��a�� д������̼������Һ���뵽��ҺA����������Ӧ�Ļ�ѧ����ʽΪ
��b�� ����1��������
��c�����������C���ϴ�Ӹ�������������Ϊ2�ˣ��Լ��������Ʒ�к��Ȼ���������������д��������̣�
��d����Ҫ����50g��������Ϊ5%���Ȼ�����Һ���������²���˳����У�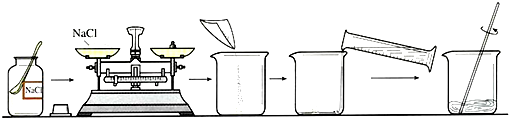
��ش��������⣺��������50g������������Ϊ5%���Ȼ�����Һ���裺
���Ȼ���
����������ƽ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣�Ӧ
A�����������Ȼ��ƹ��� B�����������Ȼ��ƹ��� C������ƽ�� D���ƶ����룮
 ��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������
��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ��������ٲ���C�п���
��Һ���ж����������
��Һ���ж����������
ʱ��ֹͣ���ȣ��ھ������������������ܷ�õ�������ʳ��
��
��
����ܡ�����2��ij������Ʒ20�ˣ����п����Ե��Ȼ������ʺͲ����Ե���ɳ�������ʺ��Բ��ƣ��ֳɶ��ȷݣ�
�ٳ����£���һ�ݸô�����Ʒ��ȫ�ܽ���һ����ˮ�У����˵õ�1.5����ɳ��
��ѧУ��ѧ��ȤС�齫��һ�ݴ�����Ʒ������һ������������NaCl��Һ��ʵ���������ͼ��������ÿһ����Ʒ����ɳ���ȵ����ķֲ���

��ش�
��a�� д������̼������Һ���뵽��ҺA����������Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+CaCl2=CaCO3��+2NaCl
Na2CO3+CaCl2=CaCO3��+2NaCl
����b�� ����1��������
����
����
������ҺB�м�������ϡ�����Ŀ������Ӧ�������̼����
��Ӧ�������̼����
����c�����������C���ϴ�Ӹ�������������Ϊ2�ˣ��Լ��������Ʒ�к��Ȼ���������������д��������̣�
��d����Ҫ����50g��������Ϊ5%���Ȼ�����Һ���������²���˳����У�

��ش��������⣺��������50g������������Ϊ5%���Ȼ�����Һ���裺
���Ȼ���
2.5
2.5
g��ˮ47.5
47.5
g������������ƽ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣�Ӧ
B
B
��A�����������Ȼ��ƹ��� B�����������Ȼ��ƹ��� C������ƽ�� D���ƶ����룮
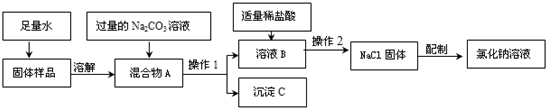
��������1���ٸ�������ʱ�IJ���Ҫ������жϣ�
�ڸ��ݹ�����ȥ�������ʳɷַ������ɣ�
��2����a������̼���ƺ��Ȼ��Ʒ�Ӧ�������д����ʽ���ɣ���b���Ӳ���1�����������������жϣ�c���ӳ����������û�ѧ����ʽ����Ȼ��Ƶ�������Ȼ�������صļ��㼴�ɣ�
��d��������һ����������������ʵ�鲽����������
�ڸ��ݹ�����ȥ�������ʳɷַ������ɣ�
��2����a������̼���ƺ��Ȼ��Ʒ�Ӧ�������д����ʽ���ɣ���b���Ӳ���1�����������������жϣ�c���ӳ����������û�ѧ����ʽ����Ȼ��Ƶ�������Ȼ�������صļ��㼴�ɣ�
��d��������һ����������������ʵ�鲽����������
����⣺��1��������ʱΪ��ֹ����ɽ������Ե���Һ�г��ֶ�������ʱ��ֹͣ���ȣ�
�ڹ�����ȥ���������Dz��������ʣ����ܳ������������ʣ����Դ�ʱ�õ���ʵ�鲻������
��2����a��̼���ƺ��Ȼ��Ʒ�Ӧ������̼��ƺ��Ȼ��ƣ��䷽��ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl��
��b���Ӳ���1���������ǹ����Һ���֪���ô��IJ����ǹ��ˣ�
��c����ʳ�����Ȼ��Ƶ�������x
Na2CO3+CaCl2=CaCO3��+2NaCl
111 100
x 2g
=
x=2.22g
��ʳ�����Ȼ��Ƶ���������=
��100%=22.2%
��d��������50g������������Ϊ5%���Ȼ�����Һ������Ȼ���Ϊ50g��5%=2.5g��ˮ��������50g-2.5g=47.5g��
��������ƽ��ָ��ƫ�����̣�˵�������أ��������Ƕ����������Ӧ���������Ȼ��ƹ��壻
�ʴ�Ϊ����1������Һ���ж���������֣�
�ڷ�
��2����a��Na2CO3+CaCl2=CaCO3��+2NaCl��
��b���Ӳ���1���������ǹ����Һ���֪���ô��IJ����ǹ��ˣ�
��c����ʳ�����Ȼ��Ƶ�������x
Na2CO3+CaCl2=CaCO3��+2NaCl
111 100
x 2g
=
x=2.22g
��ʳ�����Ȼ��Ƶ���������=
��100%=22.2%��
��ʳ�����Ȼ��Ƶ�����������22.2%��
��d����2.5g��47.5g��
��B
�ڹ�����ȥ���������Dz��������ʣ����ܳ������������ʣ����Դ�ʱ�õ���ʵ�鲻������
��2����a��̼���ƺ��Ȼ��Ʒ�Ӧ������̼��ƺ��Ȼ��ƣ��䷽��ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl��
��b���Ӳ���1���������ǹ����Һ���֪���ô��IJ����ǹ��ˣ�
��c����ʳ�����Ȼ��Ƶ�������x
Na2CO3+CaCl2=CaCO3��+2NaCl
111 100
x 2g
| 111 |
| 100 |
| x |
| 2g |
x=2.22g
��ʳ�����Ȼ��Ƶ���������=
| 2.22g |
| 10g |
��d��������50g������������Ϊ5%���Ȼ�����Һ������Ȼ���Ϊ50g��5%=2.5g��ˮ��������50g-2.5g=47.5g��
��������ƽ��ָ��ƫ�����̣�˵�������أ��������Ƕ����������Ӧ���������Ȼ��ƹ��壻
�ʴ�Ϊ����1������Һ���ж���������֣�
�ڷ�
��2����a��Na2CO3+CaCl2=CaCO3��+2NaCl��
��b���Ӳ���1���������ǹ����Һ���֪���ô��IJ����ǹ��ˣ�
��c����ʳ�����Ȼ��Ƶ�������x
Na2CO3+CaCl2=CaCO3��+2NaCl
111 100
x 2g
| 111 |
| 100 |
| x |
| 2g |
x=2.22g
��ʳ�����Ȼ��Ƶ���������=
| 2.22g |
| 10g |
��ʳ�����Ȼ��Ƶ�����������22.2%��
��d����2.5g��47.5g��
��B
������ע������ķ��뷽����̽��ʵ�������ע����������������������ƺ�ѡ�ã���ǿ�������������ļ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ


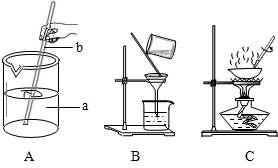 ��1�������Ƿḻ�Ļ�ѧ��Դ���⣮ͨ����ɹ��ˮ�����Եõ���������ɳ�Ĵ��Σ��������γ����ᴿ��ʵ��ʱ��Ҫ��������ͼ��ʾ��ʵ�������
��1�������Ƿḻ�Ļ�ѧ��Դ���⣮ͨ����ɹ��ˮ�����Եõ���������ɳ�Ĵ��Σ��������γ����ᴿ��ʵ��ʱ��Ҫ��������ͼ��ʾ��ʵ�������