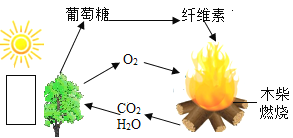
��Ŀ����
����Ŀ����ʦ�ó���ƿ�ޱ�ǩ���Լ����ֱ��ǹ����Һ�壬��ȡ�������Թ��л�ϣ���������һ����ɫ���壬�Ը�����չ��һϵ��̽����
��1����������ʲô���壿��֤����ʵ�鷽�����£�
���� | ʵ�鲽�� | ������ |
��������_____ | ______ | _______ |
��2������������Ļ�ѧ����ʽ������________________________________��
��3������ȡ�����壬ѡ�õķ���װ�ÿ�ѡȡ��ͼ�е�____���ռ�װ��Ϊ____��

��4����������廹��������һ�����壬���Բ������Ļ�ѧ��Ӧ����ʽΪ_____________________��
���𰸡� ���� �������ǵ�ľ������Թܿ� �����ǵ�ľ����ȼ��֤ �������������� 2H2O2![]() 2H2O+ O2�� B C��E CaCO3 + 2HCl = CaCl2 + H2O + CO2��
2H2O+ O2�� B C��E CaCO3 + 2HCl = CaCl2 + H2O + CO2��
����������1�������������������鷽���ǽ������ǵ�ľ������Թܿ� �������ǵ�ľ����ȼ����2������������Ļ�ѧ����ʽ��2H2O2![]() 2H2O+ O2�� ��3����Һ��Ӧ��ȡ������ѡ��װ��B��������������ˮ��ѡ��E���ܶȱȿ������ܶȴ�ѡ��C����4�������廹�����Ƕ�����̼������������̼�Ļ�ѧ��Ӧ����ʽΪ CaCO3 + 2HCl = CaCl2 + H2O + CO2��
2H2O+ O2�� ��3����Һ��Ӧ��ȡ������ѡ��װ��B��������������ˮ��ѡ��E���ܶȱȿ������ܶȴ�ѡ��C����4�������廹�����Ƕ�����̼������������̼�Ļ�ѧ��Ӧ����ʽΪ CaCO3 + 2HCl = CaCl2 + H2O + CO2��

����Ŀ������������Դ�ͱ������������ǹ�ע�����⡣��ش�
��1����ʯȼ����һ����Ҫ��Դ��������ú��ʯ�ͺ�__________��
��2��ú��Ϊȼ�ϸ���ô�Ǵ������洦�����Ի���Ҳ����˲���Ӱ�졣
�����й��������������ȷ����__________������ţ�
A. ��ʴ����ʯ���� B. ���ɶ�����̼�Ͷ������������
C. �ữ���� D. �Ժ�������û��Ӱ��
��ʹȼ�ϳ��ȼ�գ�ͨ�����˿���ȼ��ʱҪ���㹻�Ŀ�������Ҫ����___________��
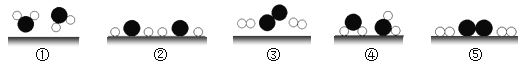
��3��Ϊ������Ⱦ�����й�����ͨ�������ƹ�ʹ��ѹ����Ȼ����Һ��ʯ����Ϊ������ij�����ȼ�ϵ���Ҫ�ɷ���A��һ��������A������B�����г��ȼ�գ����±���ʾ����
��� | A | B | C | D |
|
����ʾ��ͼ | �� |
|
|
| |
��Ӧǰ����/g | 44 | �� | 0 | 0 | |
��Ӧ������/g | 0 | 0 | 132 | 72 |
����������Ӧ���ṩ�������������Ե�����Ϊ______________����д��ѧʽ��
��A�����и�Ԫ�ص�������Ϊ________________��