��Ŀ����
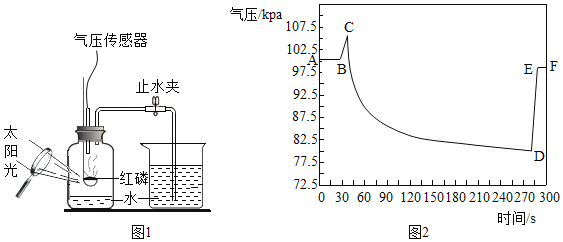
����Ŀ����ѧ��ȤС���ͬѧҪ�ⶨ�����ǣ���Ҫ�ɷ���̼��ƣ��к�CaCO3������������ȡ15g�����ǣ����飬�����ձ��У������м���80gij����������ϡ���ᣬʹ֮��ַ�Ӧ���������г�CaCO3��������ɷֶ�������ˮ���Ҳ���ϡ���ᷴӦ��������ձ��з�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ������Ӧ���е�B��ʱ����������պ������˼�������һ�롣
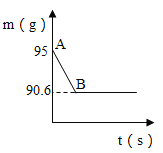
��1�����ͼ������B���ʾ��������_____��ѡ����ţ�
��CaCO3��ϡ����ķ�Ӧ�պý��е�һ�룻��CaCO3��ϡ���ᷴӦ�ѽ�������CaCO3��ȫ����Ӧ�����ձ���ʣ�����������90.6g
��2��д����������Ӧ�Ļ�ѧ����ʽ_____��
��3����Ӧ�в���CO2��������_____��
��4���ü������к�CaCO3����������Ϊ_____��
���𰸡��ڢۢ� ![]() 4.4g 66.7%
4.4g 66.7%
��������
��1��������̼��������ᷴӦ����������̼���壬�����ձ���������������٣��Ҽ�������Ϊ������̼������B���Ƿ�Ӧ����ʱ��ͼ����˷�Ӧ���е�B��ʱ��
��CaCO3��ϡ����ķ�Ӧ�պý��е�һ�룬˵������
��CaCO3��ϡ���ᷴӦ�ѽ�����˵����ȷ��
����������պ������˼�������һ�룬CaCO3��ȫ����Ӧ��˵����ȷ��
���ձ���ʣ�����������90.6g��˵����ȷ��
�����������ڢۢ���ȷ���������⡣
��2��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ����ʽΪ![]() ��
��
��3���÷�Ӧ����������̼��������Ϊ15g+80g-90.6g=4.4g��
��4���輦������̼��Ƶ�����Ϊx�����У�
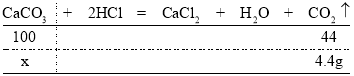
��![]() �����x=10g
�����x=10g
�ʸü������к�CaCO3����������Ϊ![]() ��
��

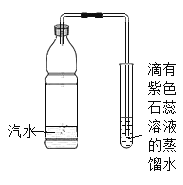
����Ŀ����ȤС����̽������ȼ������ʱ����һ������ƿ��סȼ�ŵ�С����С����һ���Ϩ���ˡ���Ϊʲô��Ϩ����?
��������룩��ͬѧ˵������ȼ�պľ���ƿ�ڵ���������������Ϩ����ͬѧ˵������ȼ�պ�ƿ�ڿ��ܻ�����������������CO2Ũ�ȹ��ߵ�������Ϩ��
��ʵ����֤��
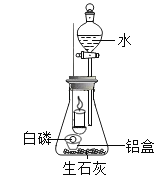
ʵ�鲽�� | ʵ������ | ʵ����� | ʵ����� |
��1��ȡһС����ף��Ż��Ϊ40����������һ�����Ƶ�С���У�Ȼ�����װ����ʯ�ҵ���ƿ�ڣ���ͼ������ȼ���� | ȼ�յ�����һ�����Ϩ���ˡ� | ����ȼ��������������� ���¶�Ҫ�ﵽ�Ż�㣻 ��_____�� | _____ͬѧ������ȷ�� |
��2������ȴ��Һ©������������ƿ��ע������ˮ�������رջ����� | �����еİ���ȼ�գ��ų�����������_____�� |
��ʵ�鷴˼��
����ʵ���У���ʯ�ҵ���Ҫ����������a._____��b._____��
��ʵ�������ͬѧ�ǶԼ���ƿ�ײ��ĺ��ƹ����ֲ�������Ȥ�����������ʵ�飺
ʵ����� | ���� | ���� |
ʵ��1��ȡ����_____ | �ų������� | �ù����к���_____ |
ʵ��2��ȡ�����μ���ɫ��̪ | _____ | �ù����к����������� |
ʵ��3��ȡ�����μ�����_____ | ������ð�� | �ù����к���̼��� |
��С�����۷��֣�ʵ��_____������ţ��Ľ��۴���������_____��
����Ŀ������Ԫ�ؿ�������������ʣ����ݱ��е�Ԫ�ػش��������⡣
Ԫ������ | �� | ̼ | �� | �� | �� |
Ԫ�ط��� | H | C | O | Ca | Fe |
��1����ij��������Է���������С������������ʵĻ�ѧʽ��_____��
��2����ij��ȼ��������������ȼ�գ����������������������һ�����е�Ԫ����_____��
��3����ijԪ����ɵĵ��ʼ�����������ܺ�X�������Ϸ�Ӧ����ͬһ�����ʡ���дһ����ط�Ӧ�Ļ�ѧ����ʽ_____��
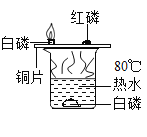
��4���ס����������ʵ�ת����ϵ����ͼ��ʾ����֪����ʹ����ʯ��ˮ����ǣ�������ɫ��Һ������ij�ֺ�ɫ�������ʴ��£����ɿɹ������������塣�����ַ�Ӧ����P��Ӧ��������ȥ����������ʾ����֮���ܷ���ת��������˵����ȷ����_____������ţ���

a�����ͼ�һ��������ͬԪ�� b�������ɼͶ�һ����Ҫ��ȼ
c����ת���ɼ��ܷ����û���Ӧ d��������Ӧһ��������������