��Ŀ����
����Ŀ��̼��̼�Ļ����������ǵ�����������ء�
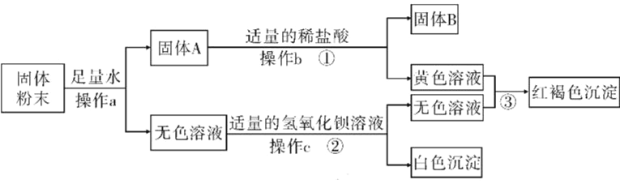
(1)��д�йغ�̼���ʵĶ�Ӧ���ԡ�
������; | ���ʯ�и�� | ʯī���缫 | ����̿��ˮ | һ����̼ұ������ |
��Ӧ���� | ��___ | ��___ | ��___ | ��___ |
(2)Һ̬������̼����������˾ȵ��������ҷ����Ļ��֣�˵����ȷ����___(����)��
A Һ̬������̼��������Ⱦ��������
B ������̼�ɸ�����ȼ������棬��������
C Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
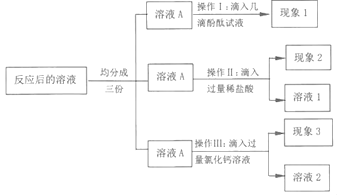
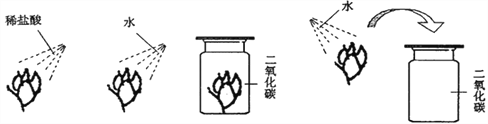
(3)����Ķ�����̼���µĻ���������___��д��һ�����ٶ�����̼�ŷŵĽ���___��
(4)������̼��һ�ֱ������Դ���ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ___��
(5)Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú��___����Ȼ�������Ƕ�����___(��������������������������)��Դ����Ȼ���м�����ȫȼ�յĻ�ѧ����ʽΪ___��
���𰸡�Ӳ�ȴ� �ܵ��� �������� �л�ԭ�� AB ����ЧӦ ��ɫ���е� CO2+2NH3![]() CO(NH2)2+H2O ʯ�� �������� CH4+2O2
CO(NH2)2+H2O ʯ�� �������� CH4+2O2![]() CO2+2H2O
CO2+2H2O
��������
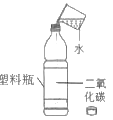
��1���ٽ��ʯ����Ȼ������Ӳ���������ʣ����Կ��ý��ʯ�и��������Ӳ�ȴ�
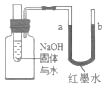
��ʯī�ܵ��磬����ʯī�������缫�������ܵ��磻
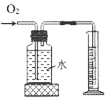
�ۻ���̿���������ԣ���������ˮ�е�ɫ�ؼ���ζ�����Ի���̿����������ˮ�������������ԣ�
��һ����̼���л�ԭ�ԣ��ܶ�ȡ�����������е��������仹ԭΪ�������ʣ����Կ���һ����̼ұ�������������л�ԭ�ԡ�
��2��A Һ̬������̼������ӷ���������Ⱦ�������ϣ�ѡ����ȷ��
B ������̼���ܶȴ��ڿ������ܶȣ��ɸ�����ȼ������棬����������ѡ����ȷ��
C��ȼ����Ż���ǿ�ȼ������ʣ������Dz���ı�ģ�ѡ�������AB��
��3�������й���Ķ�����̼����ߴ������¶ȣ���������ЧӦ����������ڻ�����ƽ�����������⣬��������ЧӦ��
Ϊ���ٶ�����̼���ŷţ���������ЧӦ�ķ��������ǿ��Բ����ٿ������ಽ�е���ɫ���з�ʽ�ȣ�������ɫ���еȡ�
��4���ڸ��¸�ѹ�£�CO2��NH3������������[CO(NH2)2]��ˮ���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��CO2+2NH3![]() CO(NH2)2+H2O��
CO(NH2)2+H2O��
��5��)Ŀǰ�������ʯ�͵Ȼ�ʯȼ��Ϊ��Ҫ��Դ������ʯ�ͣ�
��ʯȼ����Զ��ʱ���Ķ�ֲ���ź�����������������ɵģ����ڲ�����������Դ�������������
�����������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��

 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�