��Ŀ����
��������ӭ��������ͨ��չ�ĸ߳������������Ǵ����IJ����ǽ�ͨ���㣬���п�ݣ������Ǵ�������ҵ�ķ��١�����������Ҫ�����ĺϽ�֣�÷����ѧ��ѧ��ȤС��Ϊ��չ��̽��:
��һ����ҵ���������ֺ����Ƹֲĵ���Ҫ��������ͼ:

��֪:�����ĺ�̼��Ϊ2����4.3�����ֵĺ�̼��Ϊ0.03%~2%��
��1�������Ĺ���ԭ���м��뽹̿��������______________��___________���÷���ʽ��ʾ����
��2�������Ĺ���ԭ���辭�����飬��Ŀ����____________________ ��
��3���ȿ�����¯�������Ҫ�ɷ���һ����ͬ������������________���ѧʽ����
��4������¯�У�ͨ�봿�������ÿ�����Ŀ����____________________�����ֶ����ɸְ壬�����˽�����________�ԡ�
����������ȤС�鷢��δ�������ĸ����������⡣ͬѧ�ǽ���̽�����������������⡣
��֪ʶ�عˣ������������Ϊ����_______��_______ͬʱ�Ӵ���Ϊ�˷�ֹ����Ʒ���⣬Ӧ��ȡ�Ĵ�ʩ��__________��д��һ�֣���
���������ϣ�����ɷָ��ӣ���ѧʽ�ɼ�ʾΪFe2O3•xH2O���ڼ���ʱ��ֲ���Ӧ�� ����ʧȥ�ᾧˮ���䷴Ӧ�ɱ�ʾΪFe2O3•nH2O=Fe2O3��nH2OŨ���������ˮ����ʯ�ҿ�������ˮ�Ͷ�����̼��
��������⣩���⣨Fe2O3•nH2O����n��ֵ���ڶ����أ�
������̽����С������ʵ��������һ���ܲ��Ƶ����ۣ����Ѿ����ɺ��ɫ��Ϊ��̽�����⣨Fe2O3•nH2O������ɣ���ȡ27.0g����������Ʒ������ͼ2��ʾװ�ý���ʵ�顣
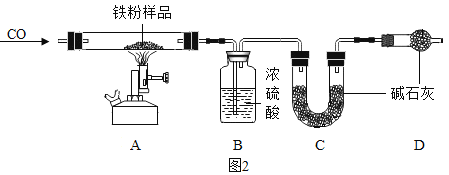
��1��ʵ��ǰӦ��_______________��
��2��Ϊ�˱�֤ʵ�鰲ȫ��ʵ�鿪ʼʱӦ��__________��Ŀ����_______________��
��3��A������� ________________��
��4����ָ����װ������һ�����Բ��� _____________��
�����ݴ�������ͼ�Ǽ���ʱ���A�й���������ϵͼ���±���B��C���������ٱ仯ʱB��Ũ���ᡢC�м�ʯ��װ�������仯�����
��Ӧǰ��g�� | ��Ӧ��g�� | |
B | 100 | 105.4 |
C | 150 | 163.2 |

��5�����⣨Fe2O3•nH2O����n��ֵ��_____��
��6��д��T3-T4ʱ��η�����Ӧ�Ļ�ѧ����ʽ _______________________��
��7������ԭ��Ʒ�е�����������������________����������������0.1����






 B.
B. C.
C. D.
D.