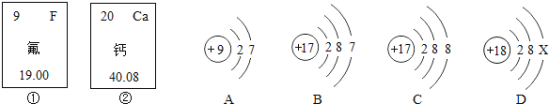��Ŀ����
����Ŀ������ͨ����˿��֮·�����й���˿��Ҷ�ȴ����������ֽ������ı�ʯ�ȴ����й������ʲ�Ҷ�����ḻ��ά����C��C6H6O6������ʯ�ijɷֺܸ��ӣ���Щ����Al2O3�ȣ�����˵����ȷ���ǣ� ��
A. һ��ά����C������21��ԭ�ӹ���
B. Al2O3��Է�������Ϊ100
C. ά����C����Ԫ������������Al2O3����Ԫ�ص�����������
D. Al2O3�н���Ԫ����ǽ���Ԫ�ص�������Ϊ27��16
���𰸡�C
��������
A���ɻ�ѧʽ��֪��ÿ��ά����C�����к���6��̼ԭ�ӡ�6����ԭ�ӡ�6����ԭ�ӣ���18��ԭ�ӣ�ѡ�����
B��Al2O3��Է�������Ϊ27��2+16��3��102��ѡ�����
C��ά����C����Ԫ�ص���������Ϊ��![]() ��64%������������Ԫ�ص���������Ϊ��
��64%������������Ԫ�ص���������Ϊ��![]() ���ɼ�ά����C����Ԫ������������Al2O3����Ԫ�ص����������ߣ�ѡ����ȷ��
���ɼ�ά����C����Ԫ������������Al2O3����Ԫ�ص����������ߣ�ѡ����ȷ��
D��Al2O3�н���Ԫ�أ�Al����ǽ���Ԫ�أ�O����������Ϊ����27��2������16��3����9��8��ѡ�����ѡC��

��ϰ��ϵ�д�
�����Ŀ