��Ŀ����
�������˾�֮�ǣ��и�֮�ݣ���1��������ǽ���Ǹ��ݴ�ͳ���ˣ�����Դ��ʫ�䡰̳������Ʈ���ڣ�����������ǽ��������ʫ�����ֳ����ӵ���Щ���� �� ����һ�㣩
��2����������Ȫ�ȼٴ����������еĺ�ȥ�����������Ȫˮ��Ӳˮ������ˮ�ļ����� ��
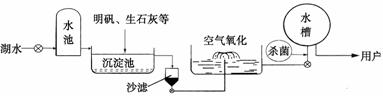
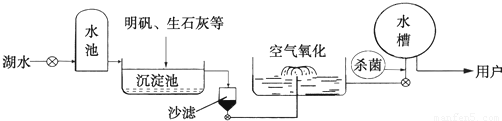
��3��ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��

��������������ˮ����ʹ�õľ�ˮ������ �������ţ�
A������ B������ C����� D������
�����������ھ�ˮ������Ϊ��������ˮ���ɽ�״��� ������ˮ�е����ʣ���������ˮ������ClO2�������� ����ȥӲˮ�й���� �������ӷ��ţ���Mg2+�Ϳ��Եõ���ˮ��
��2������Ӳˮ����ˮ�����ַ������з�����
��3��������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
�ڸ���ˮ�ľ���֪ʶ�������������ã�����������Ļ�ѧʽ�Ķ�������������Ӳˮ��ָ���н϶�����Ըơ�þ�������ˮ������ˮ��ָ�������ٺ������Ըơ�þ�������ˮ�������
����⣺��1�����ڷ����ڲ����˶�������ʳ��ķ����˶��������У����˵����ϸ���Ӵ����Ӷ��ŵ���ζ��
�ʴ�Ϊ�������Dz����˶��ģ�
��2������Ӳˮ����ˮ�ļ����ǣ�ȡ�����������ˮ���������϶��ˮ��Ӳˮ���������ˮ�����϶���ĭ��ˮ����ˮ��
�ʴ�Ϊ��ȡ�����������ˮ�������۲�����
��3��������ˮ����ʹ�õľ�ˮ�����г��������ˡ���������еȣ�
��ѡABC��
����ˮ�ľ���������������������ˮ���ɽ�״����������ʣ�ClO2�������Ƕ������ȣ���Ӳˮ����ˮ����ɿ�֪��Ӳˮ�к��н϶�ĸơ�þ���ӣ���ˮ�к��н��ٵĸơ�þ���ӣ����ӵı�ʾ��������Ԫ�ط��ŵ����Ϸ����������ĵ�ɺ͵��ԣ������ӿɱ�ʾΪ��Ca2+
�ʴ�Ϊ���������������ȣ�Ca2+
�����������������˻�ѧԴ�����Ӧ��������������ϵʵ�ʣ��龰��ӱ��������֪ʶ���ѣ��˽����֪ʶϸ�Ľ�ɣ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д��������˾�֮�ǣ��и�֮�ݡ���������ţ�������ﲻ�롰��������
��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960���� �Ĵ���������������⼣������Ҫԭ���ǣ����� �� �����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ��� ![]() ��ʩ������ǧ��Ź�����
��ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˡ�
�ٲ���Դ��ʫ�䡰̳������Ʈ���ڣ�����������ǽ��������ʫ�����ֳ����ӵ���Щ���ʣ� �� ����һ�㣩
�ڱ�1�ǡ�����ǽ�������ϱ���������������� �� �����������ʵ��� ������һ�֣�
| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |
��1��֯Ⱦ���鲼��������������ഫ����似�ա�������һ�ֲ� ��ֲ�������ά��������֯�����ɴ˿�֪�����鲼������ �����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����
����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ���� �������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ�� ������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ����� ��
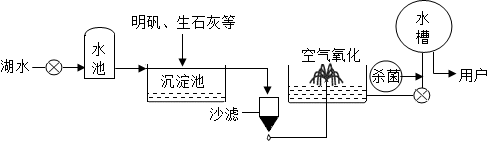
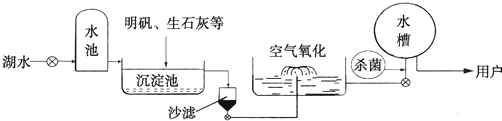
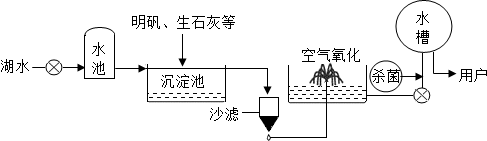
ƽ̶����컡���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ5��ʾ��
��������������ˮ����ʹ�õľ�ˮ������ �������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȡ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ
��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������
Cl2 + H2O = HClO + ��