��Ŀ����
��һ���������˾�֮�ǣ��и�֮�ݣ���������ţ�������ﲻ�롰��������

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
�ٲ���Դ��ʫ�䡰̳������Ʈ���ڣ�����������ǽ��������ʫ�����ֳ����ӵ���Щ���ʣ�______�� ����һ�㣩
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
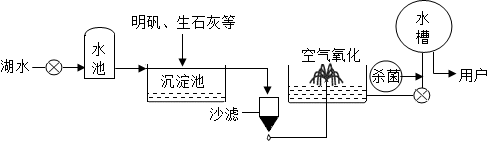
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��

��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
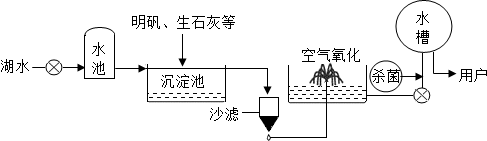
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��


��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��
��һ��
��1��������������������ˮ�����ʹ�ͬ���õĽ����
�ʴ�Ϊ��������ˮ��
��2�������ڷ����ڲ����˶�������ʳ��ķ����˶��������У����˵����ϸ���Ӵ����Ӷ��ŵ���ζ��
�ʴ�Ϊ�����Ӳ����˶���
�ڡ�����ǽ���и���������DZ��ǣ����������ʵ��м��⡢���㡢���㡢���ȣ�
�ʴ�Ϊ�����ǣ� ���⣨�����𰸾��ɣ���
������
��1�����鲼��������Ȼ��ά��
�ʴ�Ϊ����Ȼ��ά��
��2������Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����Ԫ�أ�
��ѡC��
����Һ��pH��7����Һ�����ԣ�pH��7���Լ��ԣ�pH=7�������ԣ���Ȫˮ��pH��7.5��8.9֮�䣬���Ը���Ȫˮ�Լ��ԣ�
�ʴ�Ϊ�����ԣ�
�ۼ���Ӳˮ����ˮ�ļ����ǣ�ȡ�����������ˮ���������϶��ˮ��Ӳˮ���������ˮ�����϶���ĭ��ˮ����ˮ��
�ʴ�Ϊ��ȡ�����������ˮ�������۲�����
������
������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
��ѡA B��
����ʯ�Һ�ˮ��Ӧ�����������ƣ�
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��
�����ݻ�ѧ��Ӧǰ��ԭ�ӵ�������������֪���÷�Ӧ����ʽ��Cl2+H2O=HClO+HCl��
�ʴ�Ϊ��HCl��
��1��������������������ˮ�����ʹ�ͬ���õĽ����
�ʴ�Ϊ��������ˮ��
��2�������ڷ����ڲ����˶�������ʳ��ķ����˶��������У����˵����ϸ���Ӵ����Ӷ��ŵ���ζ��
�ʴ�Ϊ�����Ӳ����˶���
�ڡ�����ǽ���и���������DZ��ǣ����������ʵ��м��⡢���㡢���㡢���ȣ�
�ʴ�Ϊ�����ǣ� ���⣨�����𰸾��ɣ���
������
��1�����鲼��������Ȼ��ά��
�ʴ�Ϊ����Ȼ��ά��
��2������Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����Ԫ�أ�
��ѡC��
����Һ��pH��7����Һ�����ԣ�pH��7���Լ��ԣ�pH=7�������ԣ���Ȫˮ��pH��7.5��8.9֮�䣬���Ը���Ȫˮ�Լ��ԣ�
�ʴ�Ϊ�����ԣ�
�ۼ���Ӳˮ����ˮ�ļ����ǣ�ȡ�����������ˮ���������϶��ˮ��Ӳˮ���������ˮ�����϶���ĭ��ˮ����ˮ��
�ʴ�Ϊ��ȡ�����������ˮ�������۲�����
������
������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
��ѡA B��
����ʯ�Һ�ˮ��Ӧ�����������ƣ�
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��
�����ݻ�ѧ��Ӧǰ��ԭ�ӵ�������������֪���÷�Ӧ����ʽ��Cl2+H2O=HClO+HCl��
�ʴ�Ϊ��HCl��

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
��һ���������˾�֮�ǣ��и�֮�ݣ���������ţ�������ﲻ�롰��������

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A�����ӡ���������B��ԭ�ӡ���������C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��

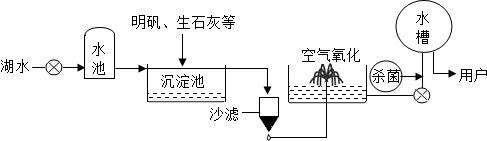
��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A����������������B�����ˡ��������� C����С���������D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��
��һ���������˾�֮�ǣ��и�֮�ݣ���������ţ�������ﲻ�롰��������

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
�ٲ���Դ��ʫ�䡰̳������Ʈ���ڣ�����������ǽ��������ʫ�����ֳ����ӵ���Щ���ʣ�______�� ����һ�㣩
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��


��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��


��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��
��һ���������˾�֮�ǣ��и�֮�ݣ���������ţ�������ﲻ�롰��������

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
�ٲ���Դ��ʫ�䡰̳������Ʈ���ڣ�����������ǽ��������ʫ�����ֳ����ӵ���Щ���ʣ�______�� ����һ�㣩
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��

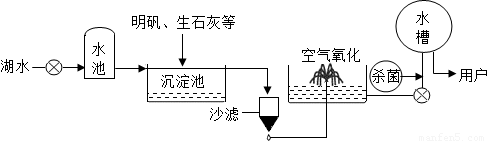
��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��

��1����ɽ�Ǹ��ݵ���Ƭ֮һ����ɽӿȪ������һ�ھ��960����Ĵ���������������⼣������Ҫԭ���ǣ�����______��______�����ʹ�ͬ���õĽ����������ȡ��Ӧ�ķ����ʩ������ǧ��Ź�����
��2��������ǽ���Ǹ��ݴ�ͳ���ˣ�
��1��������ǽ�����ϱ�
| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |
�ڱ�1�ǡ�����ǽ�������ϱ������������������______�����������ʵ���______������һ�֣�
������
��1��֯Ⱦ���鲼��������������ഫ����似�գ�������һ�ֲݱ�ֲ�������ά��������֯�����ɴ˿�֪�����鲼������______�����Ȼ��ά���ϳ���ά������
��2����������Ȫ�ȼٴ����������еĺ�ȥ����

����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����______�������ţ�
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______������ԡ��������ԡ������ԡ�����
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����______��
������ƽ̶����컣���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ������������ͼ��ʾ��


��������������ˮ����ʹ�õľ�ˮ������______�������ţ�
A������ B������ C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȣ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ______��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O=HClO+______��