��Ŀ����
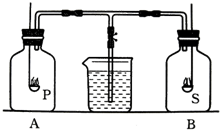 ijѧϰ�о�С��Ϊ̽����ͬ��ȼ��Բⶨ�����������������ʵ������Ӱ�죬���������ͼ��ʾ��ʵ��װ�ã�
ijѧϰ�о�С��Ϊ̽����ͬ��ȼ��Բⶨ�����������������ʵ������Ӱ�죬���������ͼ��ʾ��ʵ��װ�ã�
��֪��A��B��ֻ����ƿ�ж�������ʵ��ʱ����״̬�µĿ���������ƿ�������������ã�ȼ�ճ��зֱ�ʢ�й����ĺ�������ƿ�е�ȼ�����ȼ�պ���ȴ�����£�
��1��Aƿ�к���ȼ�յĻ�ѧ����ʽΪ______��
��2��Bƿ������ȼ��ʱ�ܹ۲쵽������Ϊ______��
��3��С���Ա���ձ��е����ϵ�ֹˮ�к۲쵽A��B��ƿ�ж�Ѹ����ˮ���������ҵ�����ƿ�е�ˮ�������ͬ����Զδ�ﵽ���֮һ�����ʵ��ʧ�ܣ�����һ�������о���С���Ա�Ƿ���ԭ���������ʵ��װ���ϣ�ֻ��Ը�װ����һ���Ķ����ܴ��ԭ�е�ʵ��Ŀ�ģ���֪������Ķ���ʲô��
______��
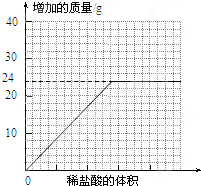
��4������ʵ��װ�ú�С���Ա�ٴν�����ʵ�飬��ʱ�ɹ۲쵽______ƿ��������Լ���֮һ�����ˮ����������һƿ��û��ˮ���������ɴ˿�֪���ⶨ�����������������ʱ��ȼ��Ӧѡ��______������ס�������
��5����ʽ���㣺��ijһ��Ӧ������������0.01mol����������Ҫ�����Ƕ���Ħ����
�⣺��1������ȼ�����������������ף���Ӧ�ķ���ʽ�ǣ�4P+5O2 2P2O5��
2P2O5��
��2��Bƿ������ȼ��ʱ�ܹ۲쵽������Ϊ����ȼ�ղ����˵���ɫ�Ļ��棻
��3����ʵ���װ�ÿ�֪��Aƿ�����ں���ȼ�����������������壬������������ѹǿ��С��Bƿ����ȼ�����˶����������壬ѹǿ���䣮������ֹˮ��ʱ��A��B����ƿ����ͨ�ģ����ԣ�A��B��ƿ�ж�Ѹ����ˮ���������ҵ�����ƿ�е�ˮ�������ͬ����Զδ�ﵽ���֮һ�����ʵ��ʧ�ܣ��ɴ˿�֪���Ľ��Ĵ�ʩ�ǣ�����ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У�
��4��������������֪������ʵ��װ�ú�С���Ա�ٴν�����ʵ�飬��ʱ�ɹ۲쵽Aƿ��������Լ���֮һ�����ˮ����������һƿ��û��ˮ���������ɴ˿�֪���ⶨ�����������������ʱ��ȼ��Ӧѡ����ף�
��5����������Ҫ�������ʵ���Ϊx
4P+5O2 2P2O5��
2P2O5��
4 5
x 0.01mol
 ��ã�x=0.008mol
��ã�x=0.008mol
�ʴ�Ϊ����1��4P+5O2 2P2O5����2����ȼ�ղ����˵���ɫ�Ļ��棻��3������ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У���4��A�����ף���5��������Ҫ������0.008Ħ����
2P2O5����2����ȼ�ղ����˵���ɫ�Ļ��棻��3������ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У���4��A�����ף���5��������Ҫ������0.008Ħ����
��������1������ȼ�����������������ף����ݷ�Ӧд����Ӧ�ķ���ʽ��
��2���������ڿ�����ȼ�յ���������ش�
��3������װ�õ��ص������������ֹˮ��ʱ��A��B����ƿ����ͨ�ģ��ݴ˷����Ķ��Ĵ�ʩ��
��4�����ݲⶨ�������������������ԭ�����������ѡ������ʣ�
��5�����ݺ���ȼ�յķ�Ӧ����ʽ���㣮
������������Ҫ�������ȼ�յķ����ⶨ��������������ʵ���ԭ���������������ۡ�ע����������⣬�Ѷ��Դ�
 2P2O5��
2P2O5����2��Bƿ������ȼ��ʱ�ܹ۲쵽������Ϊ����ȼ�ղ����˵���ɫ�Ļ��棻
��3����ʵ���װ�ÿ�֪��Aƿ�����ں���ȼ�����������������壬������������ѹǿ��С��Bƿ����ȼ�����˶����������壬ѹǿ���䣮������ֹˮ��ʱ��A��B����ƿ����ͨ�ģ����ԣ�A��B��ƿ�ж�Ѹ����ˮ���������ҵ�����ƿ�е�ˮ�������ͬ����Զδ�ﵽ���֮һ�����ʵ��ʧ�ܣ��ɴ˿�֪���Ľ��Ĵ�ʩ�ǣ�����ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У�
��4��������������֪������ʵ��װ�ú�С���Ա�ٴν�����ʵ�飬��ʱ�ɹ۲쵽Aƿ��������Լ���֮һ�����ˮ����������һƿ��û��ˮ���������ɴ˿�֪���ⶨ�����������������ʱ��ȼ��Ӧѡ����ף�
��5����������Ҫ�������ʵ���Ϊx
4P+5O2
 2P2O5��
2P2O5��4 5
x 0.01mol
 ��ã�x=0.008mol
��ã�x=0.008mol�ʴ�Ϊ����1��4P+5O2
 2P2O5����2����ȼ�ղ����˵���ɫ�Ļ��棻��3������ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У���4��A�����ף���5��������Ҫ������0.008Ħ����
2P2O5����2����ȼ�ղ����˵���ɫ�Ļ��棻��3������ͨ������ȥ�����ֱ����ӵ����ܲ���ˮ�У���4��A�����ף���5��������Ҫ������0.008Ħ������������1������ȼ�����������������ף����ݷ�Ӧд����Ӧ�ķ���ʽ��
��2���������ڿ�����ȼ�յ���������ش�
��3������װ�õ��ص������������ֹˮ��ʱ��A��B����ƿ����ͨ�ģ��ݴ˷����Ķ��Ĵ�ʩ��
��4�����ݲⶨ�������������������ԭ�����������ѡ������ʣ�
��5�����ݺ���ȼ�յķ�Ӧ����ʽ���㣮
������������Ҫ�������ȼ�յķ����ⶨ��������������ʵ���ԭ���������������ۡ�ע����������⣬�Ѷ��Դ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

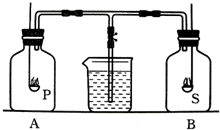 ijѧϰ�о�С��Ϊ̽����ͬ��ȼ��Բⶨ�����������������ʵ������Ӱ�죬���������ͼ��ʾ��ʵ��װ�ã�
ijѧϰ�о�С��Ϊ̽����ͬ��ȼ��Բⶨ�����������������ʵ������Ӱ�죬���������ͼ��ʾ��ʵ��װ�ã�