��Ŀ����
����Ŀ������֬�ް�סԼ0.2g�������ƣ�Na2O2����ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
��1����ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�a.���������ɣ�b.___________��
��2��ij�о�С��������ͼ��ʾװ�ý���ʵ�飬��֤���������ۡ�
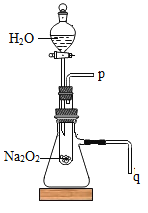
��������֤����a��ʵ����������������ǣ��������ǵ�ľ������__________����ͼ�еĵ��ܴ��ţ��ܿڣ�����Ϊ__________________________��
��������֤����b��ʵ����������������ǣ�������_________����ͼ�еĵ��ܴ��ţ�����ˮ�У�����Ϊ__________________________��
��3�����о�С���ͬѧ��ΪNa2O2��H2O��Ӧ������H2O2���������һ����ʵ��֤��Na2O2��H2O��Ӧ�����Һ����H2O2���ڡ�ʵ�������������__________________________��
���𰸡���Ӧ���� p �����ǵ�ľ����ȼ q ���ܿ�������ð�� ȡ��Ӧ�����Һ���Թ��У����������������̣����������ǵ�ľ�������Թ��� �д������ݲ�����ľ����ȼ
��������
�����Ƕԡ�����֬�ް�ס�������ƣ�Na2O2����ĩ������֬���ϵ�ˮ����֬����ȼ�յ�������̽����
��1�����ݿ�ȼ��ȼ�յ���������ȼ��Ҫȼ�ճ����������Ӵ��⣬�����¶�Ҫ�ﵽ��ȼ����Ż�㡣��ˣ�ʵ���������ó��Ľ����ǣ�a.���������ɣ�b.��Ӧ���ȡ�
��2����֤����a.���������ɵ�ʵ����������ǣ��������ǵ�ľ������p�ܿڣ�������������������Կ��������ǵ�ľ����ȼ��
��3����֤����b.��Ӧ���ȵ�ʵ����������ǣ���q��������ˮ�У���Ӧ�ų�������ʹ��ƿ��ѹǿ����ƿ�������ų������Թ۲쵽������q���ܿ�������ð����
��4�����һ����ʵ��֤��Na2O2��H2O��Ӧ�����Һ����H2O2���ڡ����˼·�����ù�������Ͷ������̷ֽ�������壬����������������֤���й������⡣ʵ������ǣ�ȡ��Ӧ�����Һ���Թ��У����������������̣����������ǵ�ľ�������Թ��У��������д������ݲ�����ľ����ȼ��֤�����������ɣ���Na2O2��H2O��Ӧ�����Һ����H2O2���ڡ�

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д�