��Ŀ����
ij��ѧ��ȤС���ͬѧ�������װ�ã��ⶨ����������ͭ��Ʒ��ͭԪ�ص��������������ʲ��μӷ�Ӧ�Ҳ�����ͭԪ�أ�����ͬ���������⣺
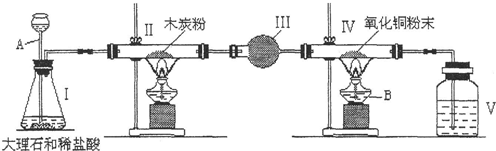
��1��ָ��������ĸ�����������ƣ�A
��2��д����װ���з����Ļ�ѧ��Ӧ����ʽ��
��3��ָ����װ�õ����ã�
��4��д��Ӳ�ʲ��ܢ��з����Ļ�ѧ����ʽ��
��5��������Ӳ�ʲ��ܢ�������Ϊ30.0g������¼���������£�
�����������ݣ�������Ʒ��ͭԪ�ص���������Ϊ
��6����ʵ���Ƿ���ȱ�ݣ����У���˵�����ɲ�����Ľ��������������˵������ͼʾ���ɣ�

��1��ָ��������ĸ�����������ƣ�A
����©��
����©��
B�ƾ���
�ƾ���
����2��д����װ���з����Ļ�ѧ��Ӧ����ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2����
CaCO3+2HCl�TCaCl2+H2O+CO2����
����3��ָ����װ�õ����ã�
����ˮ������δ��Ӧ��Ķ�����̼
����ˮ������δ��Ӧ��Ķ�����̼
����4��д��Ӳ�ʲ��ܢ��з����Ļ�ѧ����ʽ��
C+CO2
2CO
| ||
C+CO2
2CO
��
| ||
��5��������Ӳ�ʲ��ܢ�������Ϊ30.0g������¼���������£�
| ����ǰ | ��Ӧ�� | |
| Ӳ�ʲ���+��Ʒ������ | 40.0g | 38.4g |
64.0%��
64.0%��
����6����ʵ���Ƿ���ȱ�ݣ����У���˵�����ɲ�����Ľ��������������˵������ͼʾ���ɣ�
��װ��V��Ϊ���쵼�ܣ�����ȼ�ŵľƾ��ƽ������ų��������յ���
��װ��V��Ϊ���쵼�ܣ�����ȼ�ŵľƾ��ƽ������ų��������յ���
����������1����dz������������ƣ���2�����ݷ���ʽ��д�����ǣ���3����ȥһ����̼�е����ʣ���4�����ݷ�Ӧ��Ƿ����ķ�Ӧ����5�����������ļ�����������ͭ����Ԫ�ص��������ǣ���6��ȱ��β������װ�ã�
����⣺��1��Ҫ��dz������������ƺ���;��
��2����Ӧ����̼��ƺ��Ȼ��⣬���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ�������̼��������������ţ�
��3����һ����̼��ԭ����ͭʱ�����Ǵ�����һ����̼��������Ҫ��ȥ����һ����̼�е�ˮ�����Ͷ�����̼��
��4����Ӧ����̼�Ͷ�����̼����������һ����̼���ù۲취��ƽ����Ӧ�����Ǹ��£�
��5�����ݷ���ʽ��֪Ӳ�ʲ��ܢ��е������ļ�������������ͭ����Ԫ�ص�������������Ԫ������Ϊ��40.0g-38.4g=1.6g����������ͭ��ͭԪ������Ԫ�ص�������Ϊ64��16=4��1������ͭԪ������Ϊ��1.6g��4=6.4g������Ϊ��Ʒ����Ϊ��40g-30g=10g��������Ʒ��ͭԪ�ص���������Ϊ
��100%=64%��
��6������β���к���һ����̼���ж���Ⱦ����������Ҫ����β������װ�ã�
�ʴ�Ϊ����1��A������©�� B���ƾ���
��2����װ���еĻ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3����װ�õ����ã�����ˮ������δ��Ӧ��Ķ�����̼��
��4�����ܢ��еĻ�ѧ����ʽ��C+CO2
2CO��
��5��ͭԪ�ص���������Ϊ64.0%��
��6����װ��V��Ϊ���쵼�ܣ����� ȼ�ŵľƾ��ƽ������ų��������յ�����װ��V��Ϊ��
��2����Ӧ����̼��ƺ��Ȼ��⣬���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ�������̼��������������ţ�
��3����һ����̼��ԭ����ͭʱ�����Ǵ�����һ����̼��������Ҫ��ȥ����һ����̼�е�ˮ�����Ͷ�����̼��
��4����Ӧ����̼�Ͷ�����̼����������һ����̼���ù۲취��ƽ����Ӧ�����Ǹ��£�
��5�����ݷ���ʽ��֪Ӳ�ʲ��ܢ��е������ļ�������������ͭ����Ԫ�ص�������������Ԫ������Ϊ��40.0g-38.4g=1.6g����������ͭ��ͭԪ������Ԫ�ص�������Ϊ64��16=4��1������ͭԪ������Ϊ��1.6g��4=6.4g������Ϊ��Ʒ����Ϊ��40g-30g=10g��������Ʒ��ͭԪ�ص���������Ϊ
| 6.4g |
| 10g |
��6������β���к���һ����̼���ж���Ⱦ����������Ҫ����β������װ�ã�
�ʴ�Ϊ����1��A������©�� B���ƾ���
��2����װ���еĻ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3����װ�õ����ã�����ˮ������δ��Ӧ��Ķ�����̼��
��4�����ܢ��еĻ�ѧ����ʽ��C+CO2
| ||
��5��ͭԪ�ص���������Ϊ64.0%��
��6����װ��V��Ϊ���쵼�ܣ����� ȼ�ŵľƾ��ƽ������ų��������յ�����װ��V��Ϊ��

������ͨ���ش���֪��������װ��Ӧװ��ʱҪע��β����������Ҫ��Ⱦ����������������

��ϰ��ϵ�д�
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
�����Ŀ
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����


 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������