��Ŀ����
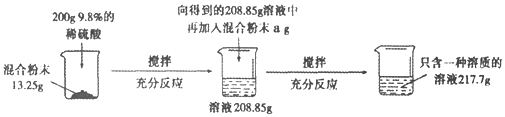
����Ŀ�������ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�˲ⶨij��������̼��Ƶ�����������СȺͬѧ����������ʵ�飺��������ϴ�������ﲢ�����ȡ10g�����ձ��Ȼ�����ձ��м���������ϡ����90g����ַ�Ӧ�Ƶ÷�Ӧʣ����Ϊ96.7g���������������ʲ������ᷴӦ��
��1������������̼������ٿˣ�
��2������ü�������̼��Ƶ�����������
��3����Ӧ��������Һ����������������
���𰸡���1��3.3g ��2��75% ��3��9%
����������1���������غ㶨�ɿ�֪���ɶ�����̼���������Ϊ10g+90g-96.7g=3.3g��
��2���輦������̼��Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
CaCO3+2HCl == CaCl2+H2O+CO2��
100 111 44
x y 3.3g
![]() =
=![]() =
=![]() ��x=7.5g��y=8.325g��
��x=7.5g��y=8.325g��
��������̼��Ƶ���������Ϊ![]() =75%��
=75%��
��3����Ӧ����Һ���Ȼ��Ƶ���������Ϊ![]() =9.0%��
=9.0%��
�������ɶ�����̼������Ϊ3.3g��̼�����Ʒ��̼��Ƶ���������Ϊ75%����Ӧ����Һ�����ʵ���������Ϊ9%��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ