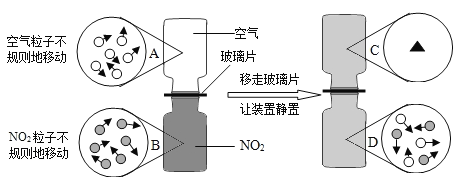
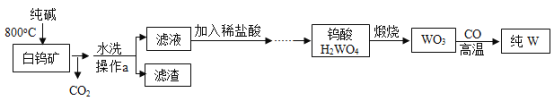
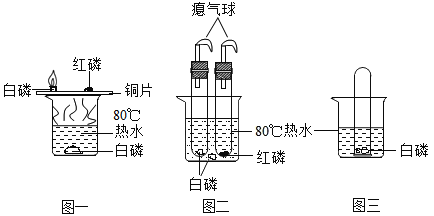
��Ŀ����
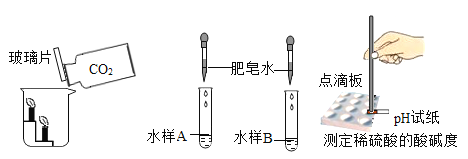
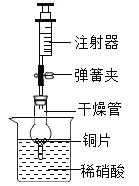
����Ŀ��ij�Ƽ�С���ͬѧ������Ȼ��Դ����˺��ɫ��ͭ�ۣ�������̿����Ϊ�˲ⶨ��ͭ����Ʒ��ͭ�������������ٷֺ�������ȡW gͭ����Ʒ���������ʵ��װ�ã�
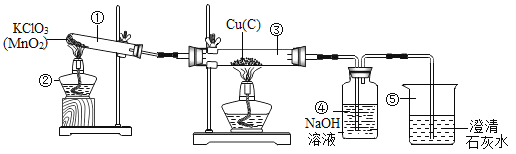
��1�������ڡ��ݵ������ǣ���_______________����______________��
��2���١����з�����Ӧ�Ļ�ѧ����ʽΪ����______________����_______________��
��3��װ�â��е�ʵ��������____________________��
��4������ʵ��ʱ����Ϩ�������ƾ��ƣ�����ȴ�����п��ܵ��µĺ���� ____________��
��5����������װ�ã�ͨ��������Ӧǰ��װ�âܵ��������õ�CO2���������������ͭ������������ʵ������к���ˮ������Ӱ�죩��Ϊ��ȷ����õ�CO2����ȷ�ɿ����ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���£�����Ϊ����Ҫ��������_____________________��
���𰸡��ƾ��� �ձ� 2KClO3![]() 2KCl+3O2
2KCl+3O2![]() 2NaOH + CO2 =Na2CO3 + H2O ���ɫ��ͭ�۱�ɺ�ɫ ��Һ�������������ܺ��Թ����� ����ر������������������NaOH��ҺҲ��������
2NaOH + CO2 =Na2CO3 + H2O ���ɫ��ͭ�۱�ɺ�ɫ ��Һ�������������ܺ��Թ����� ����ر������������������NaOH��ҺҲ��������
��������
��1�������ڡ��ݵ������ǣ��ھƾ��ƣ����ձ���
��2����������غͶ���������ȡ�������������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ��
��3��װ�â���ͭ��������Ӧ��������ͭ������ͭ�Ǻ�ɫ��
��4������ʵ��ʱ����Ϩ�������ƾ��ƣ�����ȴ������װ����ѹǿ��С�����ܵ��µĺ������Һ�������������ܺ��Թ����ѡ�
��5��Ϊ��ȷ����õ�CO2����ȷ�ɿ����ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���£����뱣֤ľ̿��ȫ����Ϊ������̼��Ҳ���뱣֤���ɵĶ�����̼����ȫ���ա�
��1�������ڡ��ݵ������ǣ��ھƾ��ƣ����ձ���
��2����������غͶ���������ȡ�������������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ����2KClO3![]() 2KCl+3O2
2KCl+3O2![]() ����2NaOH + CO2 =Na2CO3 + H2O��
����2NaOH + CO2 =Na2CO3 + H2O��
��3��װ�â���ͭ��������Ӧ��������ͭ������ͭ�Ǻ�ɫ��ʵ�������Ǻ��ɫ��ͭ�۱�ɺ�ɫ��
��4������ʵ��ʱ����Ϩ�������ƾ��ƣ�����ȴ������װ����ѹǿ��С�����ܵ��µĺ������Һ�������������ܺ��Թ����ѡ�
��5����������װ�ã�ͨ��������Ӧǰ��װ�âܵ��������õ�CO2���������������ͭ������������ʵ������к���ˮ������Ӱ�죩��Ϊ��ȷ����õ�CO2����ȷ�ɿ����ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���£����뱣֤ľ̿��ȫ����Ϊ������̼��Ҳ���뱣֤���ɵĶ�����̼����ȫ���գ�����Ҫ������������ر������������������NaOH��ҺҲ����������