��Ŀ����
ijͬѧ���������˽������̼������ˮ����ˮ������Ӧ�����ᡣ��ͬѧ���������ʵ�飬��֤����һ���ۡ�
ʵ��l��ȡһС�龭��ɫʯ����Һ���ݹ��ĸ����ֽ(���¼�ơ�ֽ��)����ֽ�ϵ��ϼ���ϡ���ᣬֽ���ɫ��ʵ��2��ȡһС��ֽ����ֽ�ϵ��ϼ��δ�����ˮ��ֽ����ɫ��
ʵ��3��
ʵ��4����������̼ͨ��ʢ��ˮ���Թ���һ������ý�ͷ���ȡ���е���Һ�ε�ֽ�ϣ�ֽ���
�ش��������⣺
��1��ʵ��2��Ŀ���ǣ� ��
��2������ʵ��3�� ��
��3��д��������̼��ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
��1��ˮ����ʹ��ɫʯ���� ��2��������ֽ����CO2�У�����ֽ����ɫ
��3��CO2 + H2O =H2CO3
�������������Ҫ̽��������̼������ˮ����ˮ������Ӧ�����ᣬ�Ǹ�����ɫʯ����Һ����������ʣ�����Ҫ��ƶԱ�ʵ���ų�����ʹ��ɫʯ����Һ�������أ����磺ˮ��������̼��
��1��ͨ����ƶԱ�ʵ��ó����ۣ�ˮ����ʹ��ɫʯ����
��2������Ҫ�ų���������� �ų�������̼�����Բ���ʵ��3��������ֽ����CO2�У�����ֽ����ɫ
��3��������̼��ˮ��Ӧ����̼�ᣬ��ѧ����ʽ��CO2 + H2O =H2CO3
���㣺������̼��ˮ��Ӧ������̽��

�����dz��л�ѧ������Ҫ��ʵ��װ�á��밴Ҫ����գ�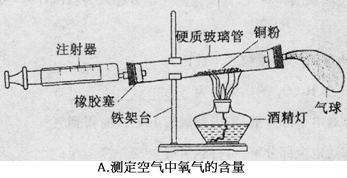
��1��A��Ϊ�˲ⶨ�����������ĺ��������û�ѧ������ȥ�����е�������������Ӧ�Ļ�ѧ����ʽ�� ����װ�����������ã�ʵ������������ĺ���С��21%����ԭ����ܢ� ���� ��
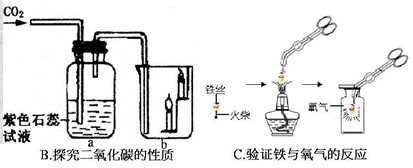
��2��Bʵ��a�п����������� ���û�ѧ����ʽ��ʾ�������ԭ�� ��bװ���е�������˵��������̼���� ���ʣ���һ������ʵ�������е�Ӧ��Ϊ ��
��3��С����Cͼʵ��ʱ������ƿ�ײ�ը�ѣ�����ܵ�ԭ���� ��
С������˿��������ȼ��Ϊʲô������������̽��������þ�Ͳ�ͬ��̼��������þ����ֱ����Ϊ0��4mm������������ȼ�գ����������¼���±��С�
| ���� | þ | ��̼0��05%���� | ��̼0��2%���� | ��̼0��6%���� |
| ȼ��ʱ ������ | ����ȼ�գ����� ҫ�۰⣬���� | ����ȼ�� ���ٻ��� | ����ȼ�� �������� | ��δ� |
�������˿��������ȼ��ʱ�����������Ҫԭ���� ��
���ж�һЩ��ʵ�Ľ��ʹ������
| | �� ʵ | �� �� |
| A | ��д�����涨����ʹ��̼��īˮ | ̼�Ļ�ѧ�����ȶ� |
| B | ������̼��ʹ������ɫʯ����Һ��ֽ����� | ������̼��ˮ��Ӧ����̼�� |
| C | ��̿�������������������ʯ�ﻹԭ���� | ��̿���������� |
| D | ���ʯ��ʯī�� C60�Ļ�ѧ�������Ƶ��������ʲ���ܴ� | ���ʯ��ʯī�� C60������̼Ԫ����ɵĵ���,�����ǵ�ԭ�����з�ʽ��ͬ |
��6�֣����ÿ���ʱ��С���ԡ�������̼���ܽ��ԡ���������Ȥ����������̽����
���������ϡ�������̼������ˮ����ͨ������£�1�����ˮԼ���ܽ�1����Ķ�����̼��������̼����ˮ�����������仯�����л�ѧ�仯��������ѧ�仯�Ļ�ѧ����ʽ�� ��
��������⡿��ʵ���������£�1�����ˮ�������ܽ��������Ķ�����̼�أ�
������ʵ�顿
�� ������ˮ��к�����ϸ��ƿ�У�����ƿ������ȴ�����£����á�������ˮ��е�Ŀ���� ��
�� ��ȡ������̼���������ſշ��ռ�����������ϲ���Ƭ�����á����������̼�Ƿ����IJ�������
�� ��
�� ʵ��٣�ȡ2֧ҽ��ע�����ֱ��ȡ10mL������10mL���õ�����ˮ���ý������ӣ���ͼ��ʾ���������ƶ�2֧ע�����Ļ�����������Ρ�
ʵ��ڣ�ȡ2֧ҽ��ע�����ֱ��ȡ10mL���ƵĶ��� ��̼�����10mL���õ�����ˮ���ý������ӣ���ͼ��ʾ���������ƶ�2֧ע�����Ļ��������������������ټ���Ϊֹ�����������ƶ�ע����������������________________________________________��
�� ��¼���ݣ�
| ʵ���� | ��ȡ�������� | ��ȡ����ˮ����� | ��ֻ�Ϻ�����Һ����� |
| | 10 mL���� | 10 mL | 20mL |
| | 10 mL���ƵĶ�����̼ | 10 mL | 12 mL |
��ע�⣺����Ա�С�⽱��4�֣���ѧ���ֲܷ�����60�֡���
����˼���ɡ�С����ʵ���ע�����ڲ������ܵ�������������ʣ����ֲ������ܵ������ǿ������Ƕ�����̼����? ���ռ�������̼ʱ����δ��ȫ�ž���Ҳ���ܵ���ʵ��ڵIJ��������ǿ���,�Ͳ������ܣ���ʵ����۲����š��������һ������̽����������ʵ������Ƿ���š�д��������衢�����ۡ�
���а�ȫ��ʩ����ȷ����
| A��ȼ���̻�����ʱ��Զ����Ⱥ�Ϳ�ȼ�� |
| B����Ȼ��й©�������رշ��Ų�����ͨ�� |
| C��ȼ�ŵľƾ��Ʋ���������������ʪ������ |
| D������ʹ�õļ��õ����Ż�������ˮ���� |
�ֱ���a��b��֧�Թ��м�����״�ʹ�С��ȫ��ͬ����Ƭ������a�м���ֲ���ͣ���������Ƥ�������Ͳ�������Ϊ��īˮ����ʼʱ����Һ��ˮƽ��������ͼ��ʾ������һ��ʱ�������˵���������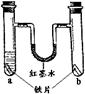
| A��ֲ�������ڸ���������ˮ |
| B����֧�Թ��е���Ƭ������ʴ |
| C�����Ͳ��������˵�Һ���Ϊ�ҵ���� |
| D�����Ͳ��������˵�Һ���Ϊ����Ҹ� |
�ֱ���a��b��֧�Թ��м�����״�ʹ�С��ȫ��ͬ����Ƭ������a�м���ֲ���ͣ���������Ƥ�������Ͳ�������Ϊ��īˮ����ʼʱ����Һ��ˮƽ��������ͼ��ʾ������һ��ʱ�������˵���������
| A��ֲ�������ڸ���������ˮ |
| B����֧�Թ��е���Ƭ������ʴ |
| C�����Ͳ��������˵�Һ���Ϊ�ҵ���� |
| D�����Ͳ��������˵�Һ���Ϊ����Ҹ� |