��Ŀ����
�����dz��л�ѧ������Ҫ��ʵ��װ�á��밴Ҫ����գ�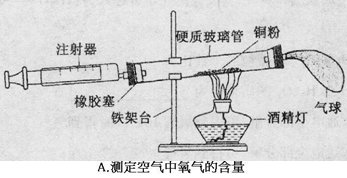
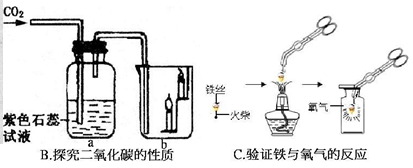
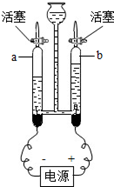
��1��A��Ϊ�˲ⶨ�����������ĺ��������û�ѧ������ȥ�����е�������������Ӧ�Ļ�ѧ����ʽ�� ����װ�����������ã�ʵ������������ĺ���С��21%����ԭ����ܢ� ���� ��
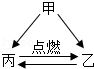
��2��Bʵ��a�п����������� ���û�ѧ����ʽ��ʾ�������ԭ�� ��bװ���е�������˵��������̼���� ���ʣ���һ������ʵ�������е�Ӧ��Ϊ ��
��3��С����Cͼʵ��ʱ������ƿ�ײ�ը�ѣ�����ܵ�ԭ���� ��
С������˿��������ȼ��Ϊʲô������������̽��������þ�Ͳ�ͬ��̼��������þ����ֱ����Ϊ0��4mm������������ȼ�գ����������¼���±��С�
| ���� | þ | ��̼0��05%���� | ��̼0��2%���� | ��̼0��6%���� |
| ȼ��ʱ ������ | ����ȼ�գ����� ҫ�۰⣬���� | ����ȼ�� ���ٻ��� | ����ȼ�� �������� | ��δ� |
�������˿��������ȼ��ʱ�����������Ҫԭ���� ��
��1��2Cu + O2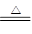 2CuO
2CuO
��ͭ�۵���̫�٣�����δ������
��δ��ȴ�����¾Ͷ�����������ע������������̫�٣�û��ʹ�ܱ����������������ͭ��Ӧ��������¶Ȳ����ߣ�ͭ����������Ӧ����ֵȵȣ�
��2����ɫʯ����Һ��Ϊ��ɫ H2O+CO2===H2CO3 ������ȼ�ա���֧��ȼ�ա��ܶȱȿ����� ���
��3������ƿ��û�з�����ˮ����һ��ϸɳ
�پ���ȼ�գ��������� ����˿�к��е�̼�����
���������������1��Ӳ�ʲ���������ͭ��ͭ����������������ͭ�Գ�ȥ�����������ͭ�۲����δ��ȴ�����¾Ͷ����������ע������������̫�٣�û��ʹ�ܱ����������������ͭ��Ӧ��������¶Ȳ����ߣ�ͭ����������Ӧ����ֵȵȾ��ᵼ�²ⶨ�������ĺ���ƫ�٣�
��2��������̼��ˮ��Ӧ����̼�ᣬ̼������ԣ���ʹ��ɫʯ����Һ��Ϊ��ɫ��b������Ϩ�����˵��������̼�Ȳ���ȼ��Ҳ����֧��ȼ�գ�
��3����ȼ��ʱ���������¶Ⱥܸߣ�������ƿ��û�з�����ˮ����һ��ϸɳ����ƿ��ը�ѣ����ݱ��������֪����̼��Խ�ߣ�ȼ��Խ���ң���ô����δ���ʵ�������Ǿ���ȼ�գ��������䡣
���㣺�����ɷ�ʵ�顢������̼����ʵ�顢��������ʵ��

 ��У����ϵ�д�
��У����ϵ�д���ͼ��ʾ�����ú�ˮ��ȡ���εĹ��̣�
��1�����ݺ�ˮɹ�ε�ԭ��������˵������ȷ�������� ��������ĸ����
| A����ˮ������ˮ�أ���ˮ�ijɷֻ������䣻 |
| B�����������У���ˮ���Ȼ��Ƶ����������ӣ� |
| C�����������У���ˮ��ˮ�����������ӣ� |
| D������������ĸҺ���Ȼ��Ƶı�����Һ�� |