��Ŀ����
��ȡ12.5gʯ��ʯ����Ҫ�ɷ���CaCO3�����ʲ��μӷ�Ӧ�������ձ��У������м���40gϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ����������ձ���ʣ�����ʵ�������Ϊ48.1g���������ձ�����������������ܽ���Բ��ƣ���
��1���Լ���ʯ��ʯ�����ʵ�����������
��2����ȡ����ʯ��ʯ12.5g����������һ��ʱ�䣬���ʣ������и�Ԫ�ص���������Ϊ40%����ʵ�ʷ�Ӧ���ɵĶ�����̼���ٿˣ�
��1���Լ���ʯ��ʯ�����ʵ�����������
��2����ȡ����ʯ��ʯ12.5g����������һ��ʱ�䣬���ʣ������и�Ԫ�ص���������Ϊ40%����ʵ�ʷ�Ӧ���ɵĶ�����̼���ٿˣ�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1�����������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ������̼������������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼�����������̼��Ƶ�����������������Ʒ�����ʵ��������������ʯ��ʯ�����ʵ�����������
��2�����Ը���̼��Ƶ���������һ�����Լ���ʯ��ʯ�и�Ԫ�ص��������ٸ���ʣ������и�Ԫ�ص��������������Լ���ʣ�������������ٸ��ݲ��������Լ������ɶ�����̼��������
��2�����Ը���̼��Ƶ���������һ�����Լ���ʯ��ʯ�и�Ԫ�ص��������ٸ���ʣ������и�Ԫ�ص��������������Լ���ʣ�������������ٸ��ݲ��������Լ������ɶ�����̼��������
����⣺
��1�����������غ㶨�ɿ�֪����CO2������Ϊ��12.5g+40 g-48.1g=4.4g
��ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
=
x=10g
������Ʒ�����ʵ�������12.5g-10g=2.5g
ʯ��ʯ�����ʵ���������Ϊ��
��100%=20%
��2��ʯ��ʯ�и�Ԫ�ص�������10t��
��100%=4g
ʣ������������
=10g
���ɶ�����̼������Ϊ��12.5g-10g=2.5g
�𰸣�
��1��ʯ��ʯ�����ʵ���������Ϊ20%
��2�����ɶ�����̼������Ϊ2.5g
��1�����������غ㶨�ɿ�֪����CO2������Ϊ��12.5g+40 g-48.1g=4.4g
��ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
| 100 |
| 44 |
| x |
| 4.4g |
x=10g
������Ʒ�����ʵ�������12.5g-10g=2.5g
ʯ��ʯ�����ʵ���������Ϊ��
| 2.5g |
| 12.5g |
��2��ʯ��ʯ�и�Ԫ�ص�������10t��
| 40 |
| 100 |
ʣ������������
| 4g |
| 40% |
���ɶ�����̼������Ϊ��12.5g-10g=2.5g
�𰸣�
��1��ʯ��ʯ�����ʵ���������Ϊ20%
��2�����ɶ�����̼������Ϊ2.5g
���������ݻ�ѧ����ʽ���м���ʱ��ֻ��ʹ�ô�������������м��㣬�����ܰѻ���������ֱ�Ӵ��뻯ѧ����ʽ���м��㣬�йط�Ӧǰ���������ٵļ����dz��л�ѧ�����һ���������ݣ�һ��Ĺ��������������ı仯���ijһ���ɵ�������

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��ȼ��һ���ǻ�ѧ�仯 |
| B�������ݳ��ֵı仯�ǻ�ѧ�仯 |
| C��������ȵı仯�������仯 |
| D��״̬�ĸı�һ���������仯 |


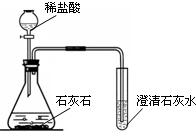 ij��ѧ��ȤС������ͼװ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������
ij��ѧ��ȤС������ͼװ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������ ��Ͳ����;����ȡҺ�����������ʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ��
��Ͳ����;����ȡҺ�����������ʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ��