��Ŀ����
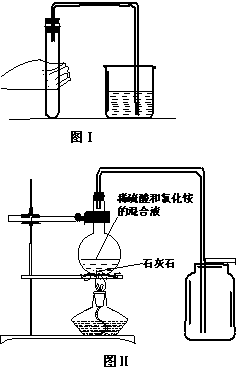
��2011?Ȫ�ݣ�ʵ����ѡ����ͼ��ʾװ����ȡ���ռ����壬����Ҫ��ش��������⣮

��1��ָ����ŵ��������Ƣ�
��2��ʵ������Bװ����ȡ����ʱ���йصĻ�ѧ��Ӧ����ʽ��
װ��B����������ȡ��������
��3��ʵ���ҳ�����ˮ�����ƺͼ�ʯ�ҵĻ�Ϲ����ڼ�����������ȡ�������壬����װ�ÿ�ѡ��
��4����װ��C�ռ�����ǰ��������ƿ�ڵĿ����ž��IJ��������ǣ�

��1��ָ����ŵ��������Ƣ�
�ƾ���
�ƾ���
����ˮ��
ˮ��
����2��ʵ������Bװ����ȡ����ʱ���йصĻ�ѧ��Ӧ����ʽ��
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
װ��B����������ȡ��������
CO2
CO2
����3��ʵ���ҳ�����ˮ�����ƺͼ�ʯ�ҵĻ�Ϲ����ڼ�����������ȡ�������壬����װ�ÿ�ѡ��
A
A
������ţ�����������һ�����װ��C�����ռ����ռ�������ƿ�ķ��÷�ʽ��ͼ2��ʾ���ݴ˿��ƶϼ���һ���߱������������ǣ�������ˮ
������ˮ
���ܶȱȿ���С
�ܶȱȿ���С
����4����װ��C�ռ�����ǰ��������ƿ�ڵĿ����ž��IJ��������ǣ�
������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
����������1������װ��ͼ��ʶ��������������ȷд�����������ƣ�
��2��ʵ�����ö������̴���������ֽ�ķ��������Լ���ʯ��ʯ��ϡ���ᷴӦ�Ʊ�������̼���н��
��3�����ݷ�Ӧ���״̬�ͷ�Ӧ��������װ�õ�ѡ��������ˮ���ռ������֪����������ˮ������˵��������ܶ�С�ڿ������ܶȣ�
��4������ˮ�ѿ����ų����н��
��2��ʵ�����ö������̴���������ֽ�ķ��������Լ���ʯ��ʯ��ϡ���ᷴӦ�Ʊ�������̼���н��
��3�����ݷ�Ӧ���״̬�ͷ�Ӧ��������װ�õ�ѡ��������ˮ���ռ������֪����������ˮ������˵��������ܶ�С�ڿ������ܶȣ�
��4������ˮ�ѿ����ų����н��
����⣺��1����ŵ��������Ƣپƾ��ƣ���ˮ�ۣ�
��2���ö������̴���������ֽ�ķ�����������ѧ��Ӧ����ʽ��2H2O2
2H2O+O2������ʯ��ʯ��ϡ���ᷴӦ�Ʊ�������̼Ҳ�����ø�װ�ã�
��3��������ˮ�����ƺͼ�ʯ�ҵĻ�Ϲ����ڼ�����������ȡ�������壬��Ӧ���״̬��Ϊ���壻��Ӧ���������ȣ�����װ�ÿ�ѡ��A������ˮ���ռ������֪����������ˮ������˵��������ܶ�С�ڿ������ܶȣ�
��4����װ��C�ռ�����ǰ��������ƿ�ڵĿ����ž��IJ��������ǣ�������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
�ʴ�Ϊ����1���ƾ��ơ�ˮ�ۣ�
��2��2H2O2
2H2O+O2���� CO2��
��3��A��������ˮ�� �ܶȱȿ���С��
��4��������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
��2���ö������̴���������ֽ�ķ�����������ѧ��Ӧ����ʽ��2H2O2
| ||
��3��������ˮ�����ƺͼ�ʯ�ҵĻ�Ϲ����ڼ�����������ȡ�������壬��Ӧ���״̬��Ϊ���壻��Ӧ���������ȣ�����װ�ÿ�ѡ��A������ˮ���ռ������֪����������ˮ������˵��������ܶ�С�ڿ������ܶȣ�
��4����װ��C�ռ�����ǰ��������ƿ�ڵĿ����ž��IJ��������ǣ�������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
�ʴ�Ϊ����1���ƾ��ơ�ˮ�ۣ�
��2��2H2O2
| ||
��3��A��������ˮ�� �ܶȱȿ���С��
��4��������ƿװ��ˮ�����ϲ���Ƭ���ٰѼ���ƿ������ˮ���У�����ƿ��ˮ��ӯ��ƿ�������ݣ�
������������Ҫ��������Ʊ��ķ�Ӧԭ��������װ�á�������ռ�װ�õ�ѡ����п��飮ѧ��ƽʱҪ��ʵ����������������������̼���������Ʊ����й��ɣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��2011?�ɶ���ʵ���ҳ���ϡ�����ʯ��ʯ��Ӧ��ȡ������̼��
��2011?�ɶ���ʵ���ҳ���ϡ�����ʯ��ʯ��Ӧ��ȡ������̼��