��Ŀ����
����Ŀ��2014��ڶ�ʮ����������ˮ��������������ˮ����Դ���������Ǿ����Դ���⣬�ӻ�ѧ���ӽǿ���
��1��������Դ����ˮ�к�����ߵĽ���Ԫ����_____����Ԫ�ط��ţ�����ҵ�ϣ��õ������״̬���Ȼ��Ƶķ����ɵõ������ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ��________________,��Ӧ����Ϊ_____��
��2����ˮ��Դ��������ˮ������_____������ĸ���ţ���
A������������B��������C�����˷�����D����ⷨ
�ù�����_____��������ѧ�仯�����������仯����
��3�������Դ�������̲��Ŵ���������ȼ��������Ҫ�ɷ�Ϊ���飩���ן�ȼ�յĻ�ѧ��Ӧ����ʽΪ��_____��
��4������Դ����ˮΪԭ�ϣ���̫��������ʹ��������·ֽ�����������������÷�Ӧ�Ļ�ѧ����ʽΪ��_____��
���𰸡�Na 2NaCl�����ڣ�![]() 2Na+Cl2�� �ֽⷴӦ B �����仯 CH4+2O2
2Na+Cl2�� �ֽⷴӦ B �����仯 CH4+2O2![]() CO2+2H2O 2H2O
CO2+2H2O 2H2O 2H2��+O2��
2H2��+O2��
��������
�Ȼ���ͨ�������ƺ������������������е�ȼ����ˮ�Ͷ�����̼��ˮ��̫��������ʹ��������·ֽ����������������
��1����ˮ�к�����ߵĽ���Ԫ������Ԫ�أ���ѧʽΪ![]() ��������Ϊ������Ԫ�صļ�ζ���������ˮ�ģ�ͨ��ˮ����Ȼѭ���������еĺ�����Ԫ�ص�������ˮ���뺣��
��������Ϊ������Ԫ�صļ�ζ���������ˮ�ģ�ͨ��ˮ����Ȼѭ���������еĺ�����Ԫ�ص�������ˮ���뺣��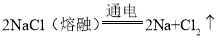 ����Ӧ���ϷֽⷴӦ��һ��������ص㣬���ڷֽⷴӦ��
����Ӧ���ϷֽⷴӦ��һ��������ص㣬���ڷֽⷴӦ��
��2�������Եõ�����������ˮ����������������ˮ����ѡB�����������û�����������ɵı仯���������仯��
��3�������������е�ȼ����ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ��Ӧ����ʽΪ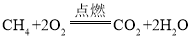 ��
��
��4����ˮΪԭ�ϣ���̫��������ʹ��������·ֽ������������������ѧ����ʽΪ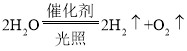 ��
��