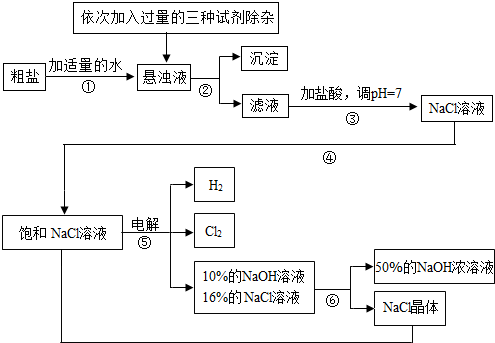
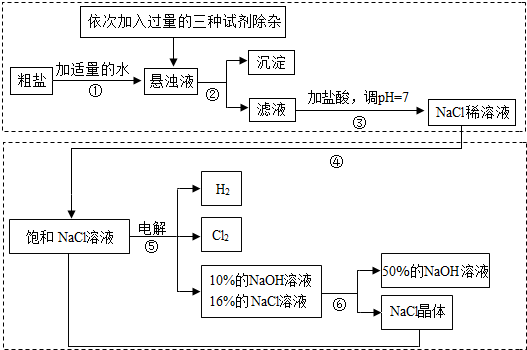
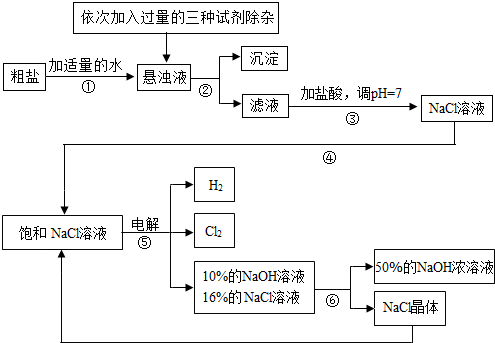
��Ŀ����
�Ե��ʳ��ˮΪ������ȡ�������������ƵȲ�Ʒ�Ĺ�ҵ��Ϊ���ȼҵ��������Ŀǰ��ѧ��ҵ����Ҫ֧��֮һ�����ڴ����к�������MgCl2��CaCl2��Na2SO4�����ʣ������ϵ��Ҫ����˱��뾭�����ơ��Դ���Ϊԭ�ϵġ��ȼҵ���������£�

�ش��������⣺
��1�����������������ڹ�ҵ���й㷺����;�����й����������Ƶ������У�������� ��
A����ȥ�����ۣ������������� B.������ˮ���ܽ�ʱ�ų���������
C��ˮ��Һ��ʹʯ����Һ��� D.������ijЩ����ĸ����
��2�������ڵ������� �������ܵ������� ��
��3�������٢ڼ������ʱ���ӵ������Լ���NaOH��Һ��Na2CO3��Һ��BaCl2��Һ��������������˳��Ҫ���ǣ�Na2CO3��Һ������BaCl2��Һ֮ ���ǰ�������롣��ͬѧ����� ��Һ����BaCl2��Һ�ɴﵽͬ����Ŀ�ġ�
��4����ⱥ��NaCl��Һ�Ļ�ѧ����ʽ�� ��
��5���������п���ѭ�����õ������� ��
��1�� C �� ��2�� ���� �� ���� ����3�� �� �� Ba(OH)2
��4��2NaCl+2H2O����![]() �� 2NaOH+H2��+ Cl2�� �� (5) NaCl
�� 2NaOH+H2��+ Cl2�� �� (5) NaCl