��Ŀ����
����Ŀ��1926�꣬�ҹ�������ѧ�Һ�°����������˺����Ƽ���ٽ����ҹ����幤ҵ�ķ�չ�������Ƽ���Ľ����������������������з�Ӧ��
��NaCl��NH3��CO2��H2O===NaHCO3��NH4Cl
��2NaHCO3![]() Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��1�������Ƽ���Ƶ���������ָ________��
��2����ҵ���������У�����ˮ���ն�����̼������̼�����ƺ��Ȼ�泥��ڳ����£��������ȴ���Һ�нᾧ��������________�����������������塣
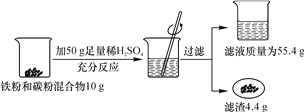
��3������Na2CO3��NaCl�Ļ������Ʒ22.3 g���������ɾ����ձ��У���һ��������ˮʹ����ȫ�ܽ⡣��������Һ����μ���������������Ϊ7.3%��ϡ���ᣬ�ձ�����Һ�����������ϡ�����������ϵ������ͼ��ʾ���Իش��������⣺
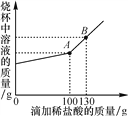
�ٵ���������ϡ������ͼ��B��ʱ���ձ�����Һ�������Ϊ________��д��ѧʽ����
����Na2CO3��NaCl�Ļ������Ʒ�У���Na2CO3������Ϊ________g��
�۵���������ϡ������ͼ��A��ʱ����ͨ�����㣬����¶�ʱ���ò�������Һ�����ʵ�����________����������ȷ��0.1 g����
���𰸡� ̼���� ̼������ NaCl��HCl 10.6 23.4 g
����������1�������Ƽ���Ƶ���������ָ����----̼���ơ�
��2���ڳ����£�̼�����Ƶ��ܽ��С���Ȼ�淋��ܽ�ȡ�
��3���ٵ���������ϡ������ͼ��B��ʱ��ϡ����ʣ�࣬�ձ�����Һ�����ɵ��Ȼ�����Һ��ʣ���ϡ���ᡣ
�ڵ���������ϡ������ͼ��A��ʱ��̼������ϡ����ǡ�÷�Ӧ����������̼���Ƶ�������x��
Na2CO3 + 2HCl == 2NaCl + H2O + CO2��
106 73
x 100g��7.3%
![]() =
=![]() �����x=10.6g
�����x=10.6g
���赱��������ϡ������ͼ��A��ʱ��̼������ϡ���ᷴӦ�����Ȼ��Ƶ�������y��
Na2CO3 + 2HCl == 2NaCl + H2O + CO2��
106 117
10.6g y
![]() =
=![]() �����y=11.7g
�����y=11.7g
ԭ��������Ȼ��Ƶ�����Ϊ��22.3 g-10.6 g=11.7g
A��ʱ��Һ���Ȼ��Ƶ�����Ϊ��11.7g+11.7g=23.4g

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������ϵ��й����������ϵ��Ȳ��ó����ٴγ�Ϊ����ʩչ���յ���̨���������ʦ�и���������:����ʱ���ּ��Ͼ��ּӴף���ʹ�˱�������ɿڣ�ԭ���Ǵ��е��������Ͼ��е��Ҵ����������������±����Ǽ��ֳ���������������������⣺
�������� | ������� | �������� | ������� | �������� |
��ѧʽ | C2H4O2 | C3H6O2 | C3H6O2 | X |
��1���������(C2H4O2)��̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ _______��
��2����������(C3H6O2)��̼Ԫ�ص���������Ϊ_______(��������ȷ��0.1%)��
��3���ȽϹ�����ѧϰ��ѧ����Ҫ�������ݱ��Ʋ�X�Ļ�ѧʽΪ________��