��Ŀ����
����Ŀ��ij��ѧ̽��С���ͬѧ����̼������Һ��ʯ��ˮ��Ӧ����ȡ�������ƣ����Ա�NaOH��Ca(OH)2���ܽ�ȡ�
��1��ʵ����̣�
�ٰ�ʯ��ˮ����ʢ��̼������Һ���ձ��г�ֽ��裬������Ӧ�Ļ�ѧ����ʽ��__
����ͨ��__�������õ���ɫ��Һ��
�۽���ɫ��Һͨ��__�������õ���ɫ���壮
��2�����룺�õ��İ�ɫ�����Ǵ�������������λͬѧ�ֱ��������²��룺
С��Ǵ������������ƣ�
�Ѽѣ����ܻ�����̼���ƣ�
Сǿ��Ҳ���ܺ���__��
��3����֤��������֤�ѼѵIJ��룮
ʵ����� | ʵ������ | ʵ����� |
ȡ������ɫ���������Һ����������__ | __ | __ |
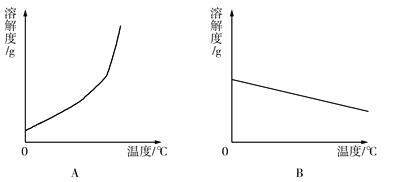
�±���Ca(OH)2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca(OH) | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
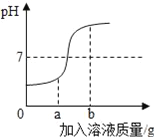
��4�������ϱ����ݣ�����Ca(OH)2��NaOH���ܽ�����ߣ���ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_________����A��B����
��5��Ҫ���һƿ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ�������ʩ�У�
�ټ����������ƣ��������¶ȣ��۽����¶ȣ��ܼ���ˮ��������ˮ���ٻָ���ԭ�¶ȣ�������ʯ�ҡ� ���д�ʩ��ȷ����_____��
A���ڢܢ� B���ۢ� C���٢ۢݢ� D���٢ڢݢ�
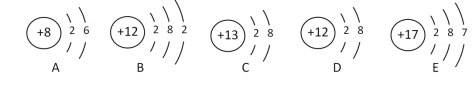
��6������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ�����ʵ��������� ��________�ף�����������������������������
���𰸡�Ca(OH)2+Na2CO3 = 2NaOH+CaCO3�� ���� ���� �������� ϡ���� ���� ����̼���� A D =
��������
��1����ʯ��ˮ�е�����������̼���Ʒ�Ӧ�����������ƺ�̼��Ƴ������ʻ�ѧ����ʽдΪCa(OH)2+Na2CO3 = 2NaOH+CaCO3����
��̼����Dz����Ե����ʣ���ѡ�ù��˽�̼�����Һ����룬���������
�۽���ɫ��Һͨ�������������õ���ɫ����������������
��2������������̼���Ʒ�Ӧ�����������
������������̼����ǡ����ȫ��Ӧ����Һ�е�����ֻ���������ƣ�
���������Ʋ��㣬��Ӧ��̼������ʣ�࣬��Һ�е��������������ƺ�̼���ƣ�
��̼���Ʋ��㣬��Ӧ������������ʣ�࣬��Һ�е��������������ƺ������������������⣬��������������
��3��̼�������ᷴӦ�ܷų�������̼���壬����Ϊ��Һ�������ɵ��������ƣ����Լ������Ӧ����������ϡ������
�����Һ����̼���ƣ�̼����������ϡ�������ɶ�����̼�������ݣ����������ݣ�
�����ݲ�����˵����Һ����̼�������ԣ������̼���ơ�
��4����ͼ�п�֪�������Ƶ��ܽ�����¶����߶���������������Ƶ��ܽ�����ߵ���A����ѡA��
��5���ټ����������ƿ���ʹ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ��ѡ����ȷ��
�������¶����������Ƶ��ܽ�ȱ�С������ʹ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ��ѡ����ȷ��
�۽����¶ȣ��������Ƶ��ܽ�ȴ���Һ���ø������ͣ�ѡ�����
�ܼ���ˮ���ܼ����࣬��Һ���ø������ͣ�ѡ�����
�ݼ����ܼ�������ʹ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ��ѡ����ȷ��
��ʵ�֢�����ʯ������ʯ�ҿ�����ˮ��Ӧ�������������������ʣ�ͬʱ��Ӧ����ˮ����������Һ�е��ܼ�������ʹ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ��ѡ����ȷ������ʹ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ�ķ������٢ڢݢޣ���ѡD��
��6����20��ʱCa(OH)2�ı�����Һ������һ����CaO����CaO��ˮ��Ӧ����Ca(OH)2 ��ͬʱ������Һ�е�һ����ˮ���൱�ڸ�������Һ�����ܼ����������壬ʣ����Һ��Ϊ���¶ȵı�����Һ�����������������ֲ�������ѡ����