��Ŀ����
����Ŀ������ʢ��ʯ��ʯ��ijУ����С��Ϊ�˽�ʯ��ʯ��Դ��Ʒ�ʣ��������ַ����Ե���ʯ�Ľ��л�ѧ������
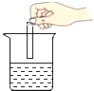
����һ��ȡ20gʯ��ʯ��ĩ��Ʒ������ͼ��ʾװ�ã���ּ����������㶨�����ʼ��Ȳ��ֽ⣩����ȴ��Ƶù�������Ϊ16.48g��
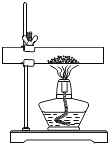
����������ȡ20gͬ�ʵ�ʯ��ʯ������Ʒ�������ձ��м�����ϡ���ᣨ���ʲ���ϡ���ᷴӦ������ַ�Ӧ��Ƶû�����������������8.36g��
��������ַ�����õ�ʯ��ʯ��̼��Ƶ�����������
��1������һ��̼��Ƶ����������� ��
��2����������̼��Ƶ�����������
��3���Ƚ������������Ľ�����������������ϴ�Ŀ���ԭ��
���𰸡���1��40%����2��95%����3�������Ǿƾ��ƻ����¶�ƫ�ͣ�ʯ��ʯ��ĩδ����ȫ�ֽ⣬�������ϴ�
����������1�����������غ㶨�ɣ�����һ�����ɵĶ�����̼������Ϊ��20g-16.48g=3.52g
��μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3 ![]() CaO+CO2��
CaO+CO2��
100 44
x 3.52g
![]()
x=8g
����Ʒ̼��Ƶ���������Ϊ![]() ��100%=40%
��100%=40%
����ʯ��ʯ����������Ҫ���£�������һ�Ǿƾ��Ƽ��ȣ��¶�ƫ�ͣ����ܻᵼ��̼��Ʋ�����ȫ�ֽ⣮
��2������ֻ��̼��ƺ����ᷴӦ�����Լ��ٵľ���̼��ƣ�����̼��Ƶ�����Ϊ8.36g������Ʒ̼��Ƶ���������Ϊ![]() ��100%=41.8%
��100%=41.8%
��3������ʯ��ʯ����������Ҫ���£�������һ�Ǿƾ��Ƽ��ȣ��¶�ƫ�ͣ��ᵼ��̼��Ʋ�����ȫ�ֽ⣬���ϴ���

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�