��Ŀ����
����Ŀ����������(CaO2)��һ�ְ�ɫ���壬����ζ���ܳ��⣬������ˮ������ˮ������Ӧ�������ڴ��ࡢ���ѵȣ������ᷴӦ��������ɱ�������������ȡ�ͨ������CaCl2�ڼ�����������H2O2��Ӧ�Ƶá�
ij��ѧ��ȤС����ʵ�����Ʊ�CaO2��ʵ�鷽�����£�

��ش��������⣺
��1���ٷ�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_____��
��2���������ϴ��CaO2��8H2O��Һ��X�����ѡ����_____��
A ��ˮ�Ҵ� B��Ũ���� C��ˮ D��CaCl2��Һ
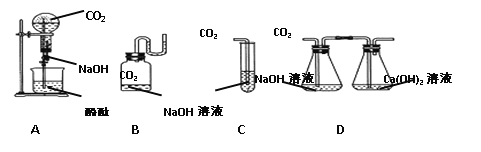
��3���������ƿ����ڳ�;�������磬�������˹�������_____�����ʣ�
A�ɻ������� B�����������������CO2����
C���ܳ��� D�����־�
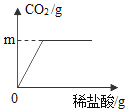
��4����֪CaO2��350��ʱ��Ѹ�ٷֽ⣬����CaO��O2����С�������ͼ��ʾ��װ�òⶨ�ղ��Ʊ��IJ�Ʒ��CaO2�Ĵ���(�����ʲ��ֽ��������)��

�ټ���װ�������Եķ����ǣ�_____��
��ȷ��ȡ0.5000g��Ʒ�������Թ��м���ʹ����ȫ�ֽ⣬�ռ���48mg���壬���Ʒ�й������Ƶ���������Ϊ_____(����4λ��Ч����)��
���𰸡�CaCl2+H2O2+2NH3H2O+6H2O�TCaO28H2O��+2NH4Cl A ABD ���Ӻ�װ�ã���ˮ����עˮ��Һ�������������γ�Һ������һ��ʱ�䣬��Һ���ֲ��䣬��װ�ò�©������֮װ��©�� 43.20%
��������
��1����ͼ��֪�ٵĻ�ѧ����ʽΪ��CaCl2+H2O2+2NH3H2O+6H2O�TCaO28H2O��+2NH4Cl��
��2�����˺��������ҪΪCaO28H2O��ϴ�ӳ���ʱ��Ҫʹ��������Һ�е��ܽ�Ⱦ�����С�����ǵ��������ƣ�CaO2�������ڴ��ࡢ���ѵȣ�����Ӧѡ����ܼ�Ϊ��ˮ�Ҵ���
��3����������Ϣ�������������ڳ�;�������磬���ܵ�ԭ���ǿ���ˮ������Ӧ�ų�����������������ɱ��������������CO2�ȣ�
��4����ʵ����������ܲ��������װ�õ������ԣ���ͨ��עˮ�۲���������ˮ����Һ������жϣ����Ӻ�װ�ã���ˮ����עˮ��Һ�������������γ�Һ������һ��ʱ�䣬��Һ���ֲ��䣬��װ�ò�©������֮װ��©����
����μӷ�Ӧ�������Ƶ�����Ϊx��

![]()
x=216mg��
��Ʒ�й������Ƶ���������=![]() 100%
100%![]() 43.20%��
43.20%��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д�