��Ŀ����
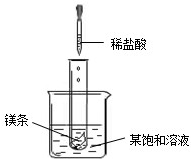
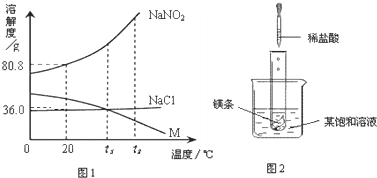
16��NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ����ͼ���֪��������

���������ݹ����ܽ�����߿��ԣ��ٲ��ij��������ij�¶��µ��ܽ�ȣ��Ӷ�ȷ��ͬ�¶��µ�һ�����ı�����Һ�����ʵ��������ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С���Ӷ��ж�ͬ�¶��µı�����Һ�����ʵ����������Ĵ�С�������жϵ������ܼ��������ܽ�����ʵĶ��٣�
����⣺A��������20��ʱ��NaNO2���ܽ����80.8g������100��ˮ������ܽ�80.8gNaNO2����A����
B���������ʵ��ܽ�����߿�֪����t2��ʱ���������ʵ��ܽ��С��ϵ��NaNO2��NaCl��M����B����
C�������Ȼ��Ƶ��ܽ�����¶ȵ����߶��������¶ȵ�Ӱ���С����M���ܽ�����¶ȵ����߶���С����˽�NaCl��M�ı�����Һ��t2�潵�µ�t1��ʱ���Ȼ�����Һ�л��������ľ�����������Һ�Ծ��DZ�����Һ����M�ı�����Һ�ͻ��ɲ�������Һ��������t2��ʱ�������ʵ��ܽ����ȣ���M����Һ�Dz�������Һ�����M��Һ�����ʵ���������ҪСһЩ����C����
D��������20��ʱ��NaNO2���ܽ�ȱ�NaCl��ܶ࣬����ڵ�����ˮ���ܽ��NaNO2�࣬��D��ȷ��
��ѡD��
B���������ʵ��ܽ�����߿�֪����t2��ʱ���������ʵ��ܽ��С��ϵ��NaNO2��NaCl��M����B����
C�������Ȼ��Ƶ��ܽ�����¶ȵ����߶��������¶ȵ�Ӱ���С����M���ܽ�����¶ȵ����߶���С����˽�NaCl��M�ı�����Һ��t2�潵�µ�t1��ʱ���Ȼ�����Һ�л��������ľ�����������Һ�Ծ��DZ�����Һ����M�ı�����Һ�ͻ��ɲ�������Һ��������t2��ʱ�������ʵ��ܽ����ȣ���M����Һ�Dz�������Һ�����M��Һ�����ʵ���������ҪСһЩ����C����
D��������20��ʱ��NaNO2���ܽ�ȱ�NaCl��ܶ࣬����ڵ�����ˮ���ܽ��NaNO2�࣬��D��ȷ��
��ѡD��
�����������ѶȽϴ���Ҫ�����˹����ܽ�����ߵ����弰���ݹ�����ܽ�����߽���йص����⣬����ѧ���������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
 ��1��KNO3��NaNO2��NaCl�������ʵ��ܽ�����¶ȵ�Ӱ��������
��1��KNO3��NaNO2��NaCl�������ʵ��ܽ�����¶ȵ�Ӱ�������� ת�����ǣ�A����Һ������ʱ�����ᾧˮ�� ��
ת�����ǣ�A����Һ������ʱ�����ᾧˮ�� ��
 ��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺
��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺