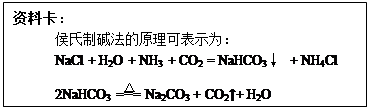
��Ŀ����
������Ϊm�˵�CaCO3��KHCO3�Ļ�����У�������������Ϊa%��ϡ����n�ˣ�ǡ����ȫ��Ӧ�õ�������̼��������w�ˡ�����֪��KHCO3 + HCl =" KCl" + H2O +CO2��������˵����ȷ����
| A���������CaCO3��KHCO3��������һ����1:1 |
| B��������ַ�Ӧ��������Һ�������ǣ�m+n��a%-w���� |
| C���������CaCO3��KHCO3�������κα�����ϣ�����ϡ�������������Ϊn�� |
| D���������CaCO3��KHCO3�������κα�����ϣ����ɶ�����̼����������Ϊw�� |
D
�����������CaCO3��KHCO3�����ᷴӦ�Ļ�ѧ����ʽCaCO3+2HCl�TCaCl2+CO2��+H2O��KHCO3+HCl�TKCl+CO2��+H2O��֪��CaCO3��KHCO3�뷴Ӧ���ɵĶ�����̼�������ȶ���100��44����ֻҪ�������������䣬���ɵĶ�����̼�������Ͳ��䣬����w�ˣ����Ի������CaCO3��KHCO3�������ȿ�Ϊ����ȣ���Ӧ��ֻ�ж�����̼����ų������Ի�����ַ�Ӧ��������Һ�������ǣ�m+n-w��g���ɻ�ѧ����ʽ��֪��̼��ƺ����ᷴӦ��������Ϊ100��73����̼����غ����ᷴӦ��������Ϊ100��36.5�����Ի������CaCO3��KHCO3�Ļ�ϱ�����ͬ��������ϡ���������������ȡ�ѡD��
��������ѧ��Ӧ���������غ㶨�ɣ������ʵ��������ǹ̶��ģ�����CaCO3��KHCO3����Է�����������ȵģ���Խ������Ժ���Ҫ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

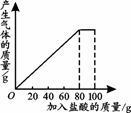

 ������22�˶�����̼��Ϊԭ�ϣ�����������ת���õ���ϩ���ٿˣ�
������22�˶�����̼��Ϊԭ�ϣ�����������ת���õ���ϩ���ٿˣ�