��Ŀ����
����Ŀ����������������Ӧ��ʮ�ֹ㷺������ұ���������ѳ�ΪĿǰ��ѧ�о��ͼ��������е���Ҫ���⣮
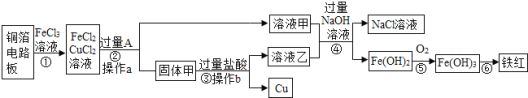
һ��������ʹ�õķ�������������������ԭ�����乤��������ʵ�����ɻ�ѧ��ת��Ϊ �ܣ�
��������ʯұ��������һ�����ӵĹ��̣�������ʯ�ͽ�̿��ʯ��ʯһ������¯���ڸ����£����ý�̿��������Ӧ���ɵ�һ����̼����������ʯ�ﻹԭ�������õ����Ͻ�һ����̼��ԭ�������Ļ�ѧ����ʽΪ ��
��1����ͬѧΪ̽�����Ͻ������������������Ⱥ�������Ĵ�ʵ�飨���ʲ���ϡ���ᷴӦ����ʵ���������±���
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
��ȡ�Ͻ�������Mg | 10 | 10 | 20 | 30 |
����ϡ����������Mg | 100 | 120 | 80 | X |
���������������Mg | 0.3 | 0.3 | 0.3 | Y |
��ش��ϱ����Ĵ�ʵ���кϽ�������ǡ����ϡ������ȫ��Ӧ����Y= g�������Ͻ����������������Ƕ��٣�д��������̣���
��2����ͬѧ�������̼Ҳ���л�ԭ�ԣ�̼��ԭ�������Ļ�ѧ����ʽΪ ��Ϊʲô��ҵ�ϲ��ý�̿��Ϊ��ԭ��ֱ�ӻ�ԭ����ʯ�����ǣ���ģ�ҵ����������¶Ա�ʵ�飨�������ϻ�֪NaOH��Һ������CO2���壩��
��ȡ������ͬ��������������ĩ��һ����������̼�۾��Ȼ�Ϸ�����ͼװ��A��Ӳ���Թ��ܣ���һ�ݷ�����ͼװ��B��Ӳ�ʲ������У�
��װ��A��B����ʵ��ͬʱ��ʼ���У���װ��B�ķ�Ӧ�У�ʵ�鿪ʼǰ���� ��ѡ�ͨCO�����ȡ�����ʵ���������ͨ��COֱ����ȴ�����£�
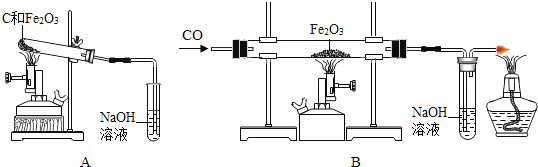
��ʱ������װ��B��Ӳ�ʲ������й�����ȫ�� ɫ��Ϊ ɫ��װ��A��Ӳ���Թ������к�ɫ���壮�������Ϊ��̿������ʯ���ǹ��壬�Ӵ����С��������ȫ��Ӧʱ��ҵ�ϲ��ý�̿��Ϊ��ԭ��ֱ�ӻ�ԭ����ʯ��һ��ԭ��
��3����ͬѧ�����������Ϸ��ֳ�������ԭ���⣬��ҵ�ϲ��ý�̿��Ϊ��ԭ��ֱ�ӻ�ԭ����ʯ����һЩ����ԭ����д�����е�һ�� ��
��4����ͬѧ����ͼBװ�ã�ȡ��ͬ�������������������ʵ�飮����ʵ���������£�
ʵ����� | ��������������/g | ������������/g |
1 | 4.00 | 2.81 |
2 | 8.00 | 7.62 |
3 | 10.00 | 7.01 |
4 | 12.00 | 8.42 |
5 | 14.00 | 9.81 |
6 | 16.00 | 11.22 |
7 | 18.00 | 12.63 |
8 | 20.00 | 14.03 |
���ϱ����Կ������е�2��ʵ��ʱ����������ʮ�ֲ��ɿ�������ȥ���������ɴ��ֽ����ԭ����Ҫ������ ��
���𰸡�һ���ȣ�������1��0.9��84%��
��2��2Fe2O3+3C![]() 4Fe+3CO2����ͨCO���죬�ڣ�
4Fe+3CO2����ͨCO���죬�ڣ�
��3�������ͽ�̿���ǹ��壬��������4����Ӧʱ��̫�̣�
��������һ��������ʹ�õķ�������������������ԭ���������������ʵ�����ɻ�ѧ��ת��Ϊ���ܣ�����ȣ�
����һ����̼��ԭ�������������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��1�����ݱ����ṩ�����ݿ��Կ�����10g�Ͻ���80g������ǡ����ȫ��Ӧ�����ϱ����Ĵ�ʵ���кϽ������ǡ����ϡ������ȫ��Ӧ����30g�Ͻ���������������Ϊ0.9g��
����������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.9g
![]()
x=25.2g
�����Ͻ���������������=![]() =84%��
=84%��
��2��̼��ԭ�������������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ2Fe2O3+3C![]() 4Fe+3CO2����
4Fe+3CO2����
��װ��A��B����ʵ��ͬʱ��ʼ���У���װ��B�ķ�Ӧ�У�ʵ�鿪ʼǰ����ͨCO���ž�װ���ڵĿ�������ֹ����ʱ������ը��һ����̼������������Ӧ�������Ͷ�����̼����۲쵽װ��B��Ӳ�ʲ������й�����ȫ�ɺ�ɫ��Ϊ��ɫ��
��3����ҵ�ϲ��ý�̿��Ϊ��ԭ��ֱ�ӻ�ԭ����ʯ��ԭ���뽹̿��״̬�йأ������ͽ�̿���ǹ��壬�����룬��������ͽ�̿���ǹ��壬�����룮
��4����ͬѧ����ͼBװ�ã�ȡ��ͬ�������������������ʵ�飮����ʵ���������£�
ʵ����� | ��������������/g | ������������/g |
1 | 4.00 | 2.81 |
2 | 8.00 | 7.62 |
3 | 10.00 | 7.01 |
4 | 12.00 | 8.42 |
5 | 14.00 | 9.81 |
6 | 16.00 | 11.22 |
7 | 18.00 | 12.63 |
8 | 20.00 | 14.03 |
���е�2��ʵ��ʱ���������ݹ���ʮ�ֲ��ɿ�����ɴ��ֽ����ԭ����Ҫ�����Ƿ�Ӧʱ��̫�̣�������û��ȫ������ԭ�������Ӧʱ��̫�̣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�