��Ŀ����
����Ŀ���������ƺ��������������ֳ����ļ��ͬѧ�����ּ����Һ�ֱ�װ���Թ�A��B��������������ͼ��ʾ������ʵ�顣
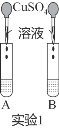
��1��ʵ��1�У���B����NaOH��Һ���μ�����ͭ��Һʹǡ����ȫ��Ӧ�����ú�۲�����Ϊ��______����ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ__________��
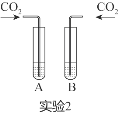
��2��ʵ��2�У���ͬѧ����֧�Թ��зֱ�ͨ�������Ķ�����̼���۲쵽A���а�ɫ�������ɣ�B��������������A�з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
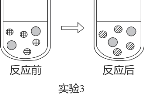
��3��ʵ��3�У�����м��η�̪������������Һ�еμ�ϡ������ǡ����ȫ��Ӧʱ����Һ�ɺ�ɫͻȻ��Ϊ��ɫ����ͼ��ʾ��Ӧǰ����Һ�д��ڵ���Ҫ���ӣ�д��ÿ��ͼ�δ��������ӡ��������ӷ��ϣ���![]() __________��
__________��![]() __________��
__________��![]() __________��
__________��
���𰸡���ɫ��״���� CuSO4+2NaOH�TCu(OH)2��+Na2SO4 Ca(OH)2+CO2=CaCO3��+H2O OH�� Ca2+ NO3��
��������
��1��ʵ��1�У���B����NaOH��Һ���μ�����ͭ��Һʹǡ����ȫ��Ӧ����������������ͭ��Ӧ����������ͭ��ɫ��״�����������ƣ����ú�۲�����Ϊ����ɫ��״��������ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪCuSO4+2NaOH�TCu(OH)2��+Na2SO4��
��2��ʵ��2�У���ͬѧ����֧�Թ��зֱ�ͨ�������Ķ�����̼��A���а�ɫ�������ɣ�˵��A��������������Һ��������Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2+CO2=CaCO3��+H2O��
��3��ʵ��3�У�����м��η�̪������������Һ�еμ�ϡ������ǡ����ȫ��Ӧʱ����Һ�ɺ�ɫͻȻ��Ϊ��ɫ��˵���μ�ǰ��Һ�к��д����ĸ����ӡ����������ӣ��μӺ���Һ�к��д����ĸ����ӡ���������ӣ���![]() �����������ӣ�
�����������ӣ�![]() �Ǹ����ӣ�
�Ǹ����ӣ�![]() ����������ӣ����OH����Ca2+��NO3����
����������ӣ����OH����Ca2+��NO3����

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�