��Ŀ����
��3�֣����ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ���������ͼ��ʾ���ش��������⣺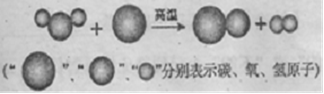
��1�������������̵���ʾ��ͼ���£����Ʊ��ϳ����Ļ�ѧ����ʽΪ ��
��2���ϳ����л�ԭ�ԣ�������ұ����������д���ϳ�����Fe2O3��Ӧ��һ����ѧ����ʽ�� ��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ��������� ������ĸ��ţ���
A.���ᣨHOOCCOOH�� B.�״���CH3OH�� C����[CO(NH2)2]
��1��C + H2O 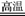 CO + H2
CO + H2
��2��3 H2+ 2Fe2O3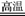 2Fe+ 3H2O ����3 CO + Fe2O3
2Fe+ 3H2O ����3 CO + Fe2O3 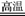 2Fe+ 3CO2
2Fe+ 3CO2
��3��C����:
��
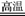 CO + H2
CO + H2 ��2��3 H2+ 2Fe2O3
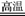 2Fe+ 3H2O ����3 CO + Fe2O3
2Fe+ 3H2O ����3 CO + Fe2O3 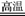 2Fe+ 3CO2
2Fe+ 3CO2 ��3��C����:
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ���������ͼ��ʾ���ش��������⣺
���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ���������ͼ��ʾ���ش��������⣺
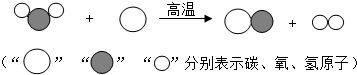

 ���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺
���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺
