��Ŀ����
 ���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ���������ͼ��ʾ���ش��������⣺
���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ���������ͼ��ʾ���ش��������⣺��1�������������̵���ʾ��ͼ���£����Ʊ��ϳ����Ļ�ѧ����ʽΪ

��2���ϳ����л�ԭ�ԣ�������ұ����������д���ϳ�����Fe2O3��Ӧ��һ����ѧ����ʽ��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʣ����úϳ���Ϊԭ�ϲ����ܵõ���������
A�����ᣨHOOCCOOH�� B���״���CH3OH�� C������[CO��NH2��2]
��4����úֱ����ȼ�ϵ���ú��ȡ�Ҷ�������õ�����ʾ��
��������1������ͼʾ����ص����ʵĽṹ�ж��仯ѧʽ����д����صķ���ʽ��
��2�����ںϳ�����������һ����̼�����л�ԭ�Զ����û����������е�����
��3�����������غⶨ�ɵ�Ӧ�ý����⣮
��4�������ֻ�ѧ֪ʶ����Ҫ�Է������ɣ�
��2�����ںϳ�����������һ����̼�����л�ԭ�Զ����û����������е�����
��3�����������غⶨ�ɵ�Ӧ�ý����⣮
��4�������ֻ�ѧ֪ʶ����Ҫ�Է������ɣ�
����⣺��1����ͼʾ��֪��Ӧǰ��ˮ�ķ�����̼ԭ�ӷ�Ӧ����һ����̼�ķ������������ӣ�����д����ѧ����ʽ��
��2��������һ����̼�����л�ԭ�Զ����û����������е�����ͬʱ����ˮ�Ͷ�����̼����Ϸ���ʽ����д������ɣ�
��3���������غ㶨�ɿ�֪��ѧ��Ӧǰ��Ԫ�ص�����䣬��֪��ӦǰԪ�������ּ�̼��������Ӧ����ֵ�Ԫ�أ��������ز����ܲ�����
��4����úֱ����ȼ�ϵ���ú��ȡ�Ҷ�������һ�ֽ��ܻ����ķ�ʽ����������˻�ѧ֪ʶ����Ҫ�ԣ�
�ʴ�Ϊ����1��C+H2O
CO+H2��2��Fe2O3+3H2
2Fe+3H2O��Fe2O3+3CO
2Fe+3CO2��3��C��4����ѧ��ָ���������������Դ������������Ҳ�ɣ�
��2��������һ����̼�����л�ԭ�Զ����û����������е�����ͬʱ����ˮ�Ͷ�����̼����Ϸ���ʽ����д������ɣ�
��3���������غ㶨�ɿ�֪��ѧ��Ӧǰ��Ԫ�ص�����䣬��֪��ӦǰԪ�������ּ�̼��������Ӧ����ֵ�Ԫ�أ��������ز����ܲ�����
��4����úֱ����ȼ�ϵ���ú��ȡ�Ҷ�������һ�ֽ��ܻ����ķ�ʽ����������˻�ѧ֪ʶ����Ҫ�ԣ�
�ʴ�Ϊ����1��C+H2O
| ||
| ||
| ||
�����������ǶԾ������Դ���õĿ��飬����Ĺؼ��Ƿ��ֲ��ҵ���Ŀ����ѧ��Ϣ�Ľ�ϵ㣬�ѶȲ���֪ʶ�������������Խ�ǿ��

��ϰ��ϵ�д�
 ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ
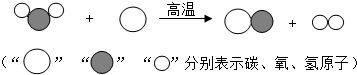

 ���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺
���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺
