��Ŀ����
������1����ѧ�����ɼ����Ŀ�״ʯ��ʯ��Ʒ��ˮ��ϴ�����ɣ��Ƶ���Ʒ����Ϊ25.0g���ð�ס�������ͬѧ��������25.0gʯ��ʯ��Ʒ�ֱ����������ʵ�飮��������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ���
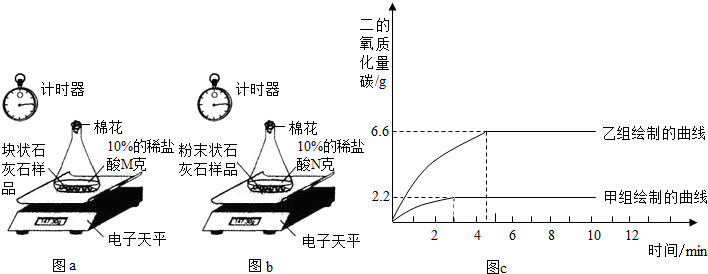
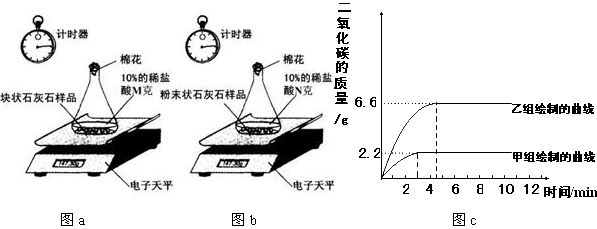
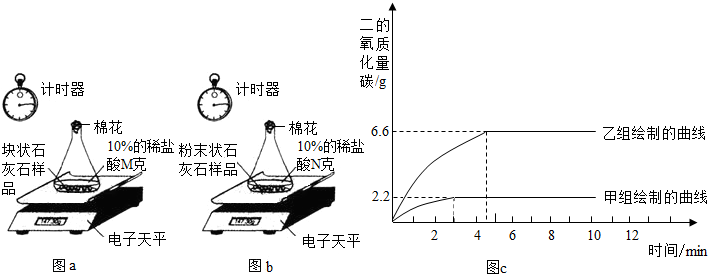
ʵ����̣����飺ȡһ�������Ŀ�״ʯ��ʯ��Ʒ����ƿ�ڣ����������10%��ϡ����Mg���ⶨ��Ӧ��������ƿ�е�ҩƷ�����仯������ͼa��
���飺��ʣ��Ŀ�״ʯ��ʯ��Ʒ����ɷ�ĩ״��Ȼ��ȫ��������һ��ƿ�ڣ�����10%��ϡ����Ng���ⶨ��Ӧ��������ƿ��ҩƷ�������仯������ͼb��
����ͬѧ�����ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵ��ͼc��
�������ۣ�
��1���ס�������ͬѧ��ʵ���У�����ʵ�����ĵ�ʱ����̣�
��2������ʵ�������ĵ�ϡ���������ȼף���=1��3��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�����������
ʵ����̣����飺ȡһ�������Ŀ�״ʯ��ʯ��Ʒ����ƿ�ڣ����������10%��ϡ����Mg���ⶨ��Ӧ��������ƿ�е�ҩƷ�����仯������ͼa��
���飺��ʣ��Ŀ�״ʯ��ʯ��Ʒ����ɷ�ĩ״��Ȼ��ȫ��������һ��ƿ�ڣ�����10%��ϡ����Ng���ⶨ��Ӧ��������ƿ��ҩƷ�������仯������ͼb��
����ͬѧ�����ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵ��ͼc��

�������ۣ�
��1���ס�������ͬѧ��ʵ���У�����ʵ�����ĵ�ʱ����̣�
��2������ʵ�������ĵ�ϡ���������ȼף���=1��3��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��1������ͼc��֪����ʵ�����ĵ�ʱ�䣨Լ3���ӣ������飨����4���ӣ����̣������
��2�������ʵ�������ĵ����������Ϊx������ʵ�������ĵ����������Ϊ y����
2HCl+CaCO3=H2O+CO2��+CaCl2
71 44
x 2.2g ����
y 6.6g ����
����
�T
�T
���� 1��3��
��3����ÿ����Ʒ��CaCO3������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x ��2.2g+6.6g��
44x=100����2.2g+6.6g��
x=20g
������Ʒ��CaCO3��������Ϊ��
��100%=80.0%��1�֣�
����Ʒ��CaCO3��������Ϊ80.0%��0.5�֣�
��2�������ʵ�������ĵ����������Ϊx������ʵ�������ĵ����������Ϊ y����
2HCl+CaCO3=H2O+CO2��+CaCl2
71 44
x 2.2g ����
y 6.6g ����
����
| x |
| y |
| 2.2 |
| 6.6 |
| 1 |
| 3 |
���� 1��3��
��3����ÿ����Ʒ��CaCO3������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x ��2.2g+6.6g��
44x=100����2.2g+6.6g��
x=20g
������Ʒ��CaCO3��������Ϊ��
| 20 |
| 25 |
����Ʒ��CaCO3��������Ϊ80.0%��0.5�֣�

��ϰ��ϵ�д�
�����Ŀ