��Ŀ����
����Ŀ��ˮ�����ǵ������������еĹ�ϵ��
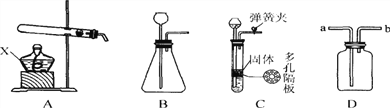
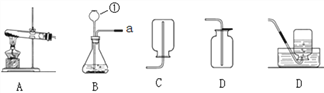
(1)Ϊ��֤ˮ���������ͼ��ʾװ�ý���ʵ�顣
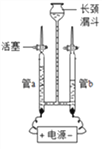
��д���÷�Ӧ�Ļ�ѧ����ʽ___________________________��
�ڵ��ˮ�Ĺ����У������ı������___________(д����)��
��������������ˮ���ܵ������_______(����ĸ)��
A������ B���Ȼ��� C���Ȼ��� D����������
(2)����200g��������Ϊ5%������������Һ����Ҫ�������Ƶ�����Ϊ______����Ҫˮ_____mL������ʱ�õ�����������_____���ձ����������ͽ�ͷ�ιܡ�(ˮ���ܶȽ��ƿ���1g/cm3)
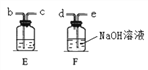
(3)���������������Һ��ij������Һ��Ӧ����ͼ���������ֻ��������õ�����ҺpH�仯ͼ��
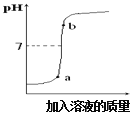
�ٸ���ͼ�����ߣ��жϽ��еIJ�����_______(����ĸ)��
A����������Һ��εμӵ�����������Һ��
B��������������Һ��εμӵ�������Һ��
�ڴ��۽Ƕȷ������÷�Ӧʵ��Ϊ_______________ ��
��b���Ӧ����Һ�е�����Ϊ________________д��ѧʽ)��
(4)����������������Ϊ9.8%��������Һ50g��������п��ȫ��Ӧ������ܵõ�������������__________________��(���ڴ����д���������)��
���𰸡� 2H2Oͨ�� 2H2��+O2�� ˮ���� BCD 10g 190 ��Ͳ B �����Ӻ����������ӽ������ˮ����(��H+��OH-���Խ�ϳ�ˮ����) Na2SO4 NaOH 0.1g
��������(1) ��ˮ��ͨ�������·ֽ������������������÷�Ӧ�Ļ�ѧ����ʽΪ�� 2H2Oͨ�� 2H2��+O2��������ѧ��Ӧ�з��ӿɷ���ԭ�Ӳ��ɷ������ˮ�Ĺ����У������ı������ˮ������ ����������ˮ���ܵ��������������A����������ˮ������Һ�������Ƿ��Ӻ�ˮ������ �����磻 B���Ȼ�������ˮ������Һ���������Ӻ����������ܵ����� C���Ȼ�������ˮ�������������Ӻ����������ܵ����� D��������������ˮ���������Ӻ��������������ܵ�����(2)����200g��������Ϊ5%������������Һ����Ҫ�������Ƶ�����Ϊ200g��5%=10g����Ҫˮ200g-10g=190g,��190mL������ʱ�õ�������������Ͳ���ձ����������ͽ�ͷ�ι���
(3) �پ�ͼ�����߿�֪����Ӧ����Һ��pH��С��7��������������7�����жϽ��еIJ����ǽ�����������Һ��εμӵ�������Һ�����ڴ��۽Ƕȷ������÷�Ӧʵ��Ϊ�����Ӻ����������ӽ������ˮ����(��H+��OH-���Խ�ϳ�ˮ����)����b�㣬���������Ӧ��Һ�е�����ΪNa2SO4 ��NaOH��(4)��:�������Ͽ��Եõ�����������ΪX
Zn+H2SO4==ZnSO4+H2��
98 2
50g��9.8% X
98/2=50g��9.8%/X
x=0.1g
��: �����Ͽ��Եõ�����������Ϊ0.1g��

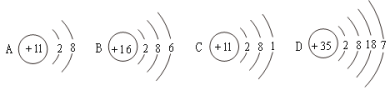
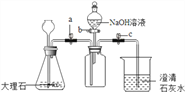
����Ŀ����ͼ�������ԭ��ģ��ʾ��ͼ��
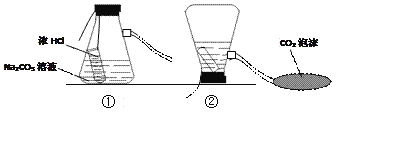
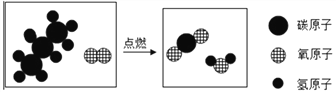
��1������װ�âڷ�����Ӧ�Ļ�ѧ����ʽ��_______________________��
��2��CO2����ԭ����_______________________��
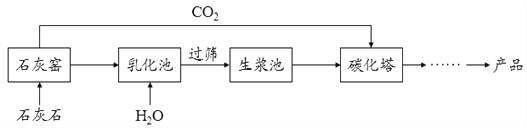
��3��ijͬѧ��װ�âڷ�Ӧ�����Һ�����ʵijɷֽ���̽����
���������ϣ��Ȼ�����������
��������룩װ�âڷ�Ӧ�����Һ������Ϊ��
I.________��II.HCl��NaCl��III.Na2CO3��NaCl��
������ʵ�飩
ʵ��װ�ü����� | ʵ����������� |
a.ȡװ�âڷ�Ӧ�����Һ���Թ��У���������ϡHNO3�� b.������a����Һ�еμ�_______�� | c.���������������1������II�������� d.__________����Ӧ�Ļ�ѧ����ʽ��________________������III������ |
����չ��˼�����������II�����������˽����ԭ����ʵ��ʱ____________________����Ӧ����Һ���ʲ����ܳ��ֵ������NaCl��Na2CO3��HCl��ԭ����__________��