��Ŀ����
��������Na2CO3��Һ���뵽һ����CuSO4��Һ�еõ���ɫ���壮ij�о���ѧϰС�����ɫ����ijɷֽ���������̽������������и��⣺��һ����������裺
����һ������ΪCuCO3�����ɣ�Na2CO3+CuSO4=CuCO3��+Na2SO4
�����������ΪCu��OH��2�����ɣ�Na2CO3��Һ��______����ᡱ����С����ԣ�
������������ΪCu��OH��2��CuCO3�Ļ���
���������ϲ��ģ�
��Cu��OH��2��CuCO3��������ˮ���侧��������ᾧˮ��
��Cu��OH��2��CuCO3���Ⱦ��ֽ⣬�����ɶ�Ӧ�����������
����ˮCuSO4Ϊ��ɫ������ˮ����ɫ����CuSO4?5H2O
�����������ʵ�飺
����Ļ�ȡ��
��1������Ӧ��Ĺ̡�Һ����ᆳ______��ϴ�ӡ����º�ɵ���ɫ���壮
��2���жϹ�����ϴ���ķ���������______��
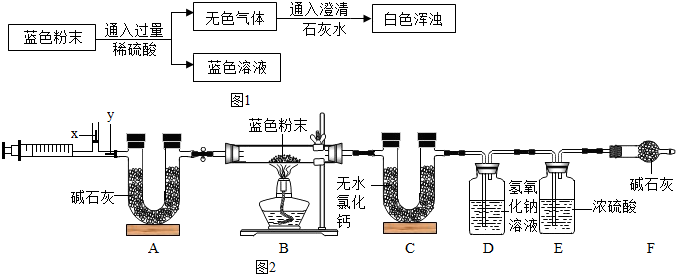
����ͼ1��ʾװ�ã�����̽������ijɷ֣�
��3������װ��A��B��Ͻ���ʵ�飬B�������������______��ȷ��
��4��С��ͬѧ��װ�ð� A��______��______���B������C������˳����Ͻ���ʵ�飬��֤������������ȷ�ģ�ʵ���У�B�е�����Ϊ______��C�а�ɫ�������ɫ��
���ۣ�����ΪCu��OH��2��CuCO3�Ļ���
����ɷֶ����ⶨ��
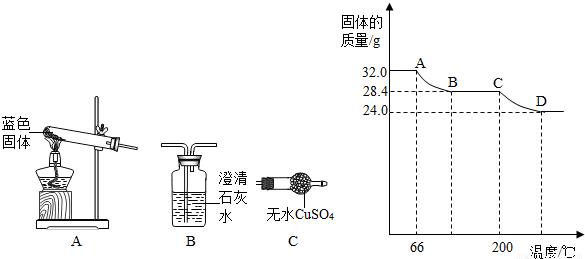
��֪Cu��OH��2�ķֽ��¶�Ϊ66���68�棬CuCO3�ķֽ��¶�Ϊ200���220�森���������ΪaCu��OH��2?bCuCO3��С��ͬѧ����
�����ǶԹ�������ȷֽ⣬���������ݣ���ɹ��������仯��ֽ��¶ȵĹ�ϵ��ͼ�������ͼ2ʾ�ش��������⣺
��5��д��A B��CD�η�����Ӧ�Ļ�ѧ����ʽ��AB�Σ�______ CuO+H2O
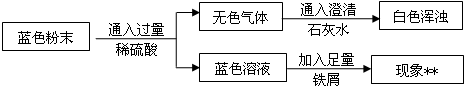
���𰸡���������һ����������裺����̼������ˮ��ˮ��IJ�����з�����
�����������ʵ�飺
��1�����ݹ����ܳ�ȥ���������ʽ��н��
��2������̼���ƻ������ᷴӦ���ɶ�����̼���з�����
��3�������������ƻ��������̼��Ӧ����̼��Ƴ������з�����
��4��������ˮ����ͭ��ˮ�������з�����
��5�����ݼ���ˮҪ���ڼ��������̼��ǰ����з�����
��6������������ͭ��̼��ͭ�ķֽ��¶Ƚ��з���������������ͭ��̼��ͭ�ֽ�Ļ�ѧ����ʽ���з�����
��7��ʣ�������ȵ������¶ȹ���������ֻ���٣���������ͭ������������Ԫ�ؿɲ������������ͭ�е���Ԫ��ת��Ϊ���������ݼ���ᷢ������ͭ����Ԫ�ص��������ڹ�����ٵ���������ô�Ϳ���������ͭ�еIJ�����Ԫ��ת��Ϊ������
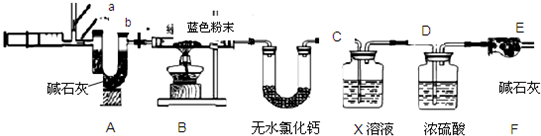
����⣺��һ����������裺̼������ǿ�������Σ���ˮ��ˮ�����������������ӣ��ʴ�Ϊ���
�����������ʵ�飺
��1�������ܳ�ȥ���������ʣ�����Ӧ��Ĺ̡�Һ����ᆳ���ˡ�ϴ�ӡ����º�ɵ���ɫ���壻�ʴ�Ϊ�����ˣ�
��2�������ϲ�����̼���ƻ������ᷴӦ���ɶ�����̼���壻�ʴ�Ϊ��ȡ���ϴ��Һ�������μ�������ϡ���ᣬ�����ݲ�����
��3��B��������˵��û�����ɶ�����̼��Ҳ��˵��������û��̼��ͭ���ʴ�Ϊ������
��4������ˮҪ���ڼ��������̼��ǰ�棬����ͨ��ʯ��ˮʱЯ����ˮ������Լ���ˮ�������ţ�ʵ���У�B�е�����Ϊ����ʯ��ˮ����ǣ�C�а�ɫ�������ɫ���ʴ�Ϊ��C��B������ʯ��ˮ����ǣ�
��5��AB�ε��¶ȴ���60�浫��û�г���200�棬��ͼ���п���Ҳ������100�棬�������������ķֽ��¶ȣ��ʴ�Ϊ��Cu��OH��2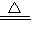 CuO+H2O��
CuO+H2O��
CD�ε��¶ȳ�����200�棬����̼��ͭ�ķֽ��¶ȣ��ʴ�Ϊ��CuCO3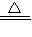 CuO+CO2����
CuO+CO2����
��6��AB����������ͭ�ڷֽ⣬������������3.6�ˣ�CD����̼��ͭ�ڷֽ⣬������������4.4�ˣ�
Cu��OH��2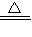 CuO+H2O CuCO3
CuO+H2O CuCO3 CuO+CO2��
CuO+CO2��
�ӷ���ʽ���Կ���һ��������ͭ�ֽ������һ��ˮ��һ��̼��ͭ�ֽ�����һ��������̼����AB�ε�������ͭ������3.6��ˮ��������0.2��ˮ����Ҫ0.2��������ͭ��CD�ε�̼��ͭ�ֽ������4.4�˶�����̼���൱�ڼ�����0.1��������̼����Ҫ0.1��̼��ͭ������a��b=2��1���ʴ�Ϊ��a��b=2��1��
��7������ͭ���Ⱥ������ֻ���٣�Ӧ��������ͭ�е���Ԫ��ת��Ϊ������24g����ͭ����Ԫ�ص�����=24g×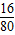 =4.8g������2.4g��˵��������ͭ�еIJ�����Ԫ��ת��Ϊ������
=4.8g������2.4g��˵��������ͭ�еIJ�����Ԫ��ת��Ϊ������
�ʴ�Ϊ��4CuO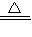 2Cu2O+O2����
2Cu2O+O2����
�������ڽ������ʱ������Ҫ�����е�֪ʶ��֪��Ȼ����ѧ����֪ʶ���н�𣬴������ѶȽϴ�Ҫϸ�Ľ��з������
�����������ʵ�飺
��1�����ݹ����ܳ�ȥ���������ʽ��н��
��2������̼���ƻ������ᷴӦ���ɶ�����̼���з�����
��3�������������ƻ��������̼��Ӧ����̼��Ƴ������з�����
��4��������ˮ����ͭ��ˮ�������з�����
��5�����ݼ���ˮҪ���ڼ��������̼��ǰ����з�����
��6������������ͭ��̼��ͭ�ķֽ��¶Ƚ��з���������������ͭ��̼��ͭ�ֽ�Ļ�ѧ����ʽ���з�����
��7��ʣ�������ȵ������¶ȹ���������ֻ���٣���������ͭ������������Ԫ�ؿɲ������������ͭ�е���Ԫ��ת��Ϊ���������ݼ���ᷢ������ͭ����Ԫ�ص��������ڹ�����ٵ���������ô�Ϳ���������ͭ�еIJ�����Ԫ��ת��Ϊ������
����⣺��һ����������裺̼������ǿ�������Σ���ˮ��ˮ�����������������ӣ��ʴ�Ϊ���
�����������ʵ�飺
��1�������ܳ�ȥ���������ʣ�����Ӧ��Ĺ̡�Һ����ᆳ���ˡ�ϴ�ӡ����º�ɵ���ɫ���壻�ʴ�Ϊ�����ˣ�
��2�������ϲ�����̼���ƻ������ᷴӦ���ɶ�����̼���壻�ʴ�Ϊ��ȡ���ϴ��Һ�������μ�������ϡ���ᣬ�����ݲ�����
��3��B��������˵��û�����ɶ�����̼��Ҳ��˵��������û��̼��ͭ���ʴ�Ϊ������
��4������ˮҪ���ڼ��������̼��ǰ�棬����ͨ��ʯ��ˮʱЯ����ˮ������Լ���ˮ�������ţ�ʵ���У�B�е�����Ϊ����ʯ��ˮ����ǣ�C�а�ɫ�������ɫ���ʴ�Ϊ��C��B������ʯ��ˮ����ǣ�
��5��AB�ε��¶ȴ���60�浫��û�г���200�棬��ͼ���п���Ҳ������100�棬�������������ķֽ��¶ȣ��ʴ�Ϊ��Cu��OH��2
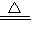 CuO+H2O��
CuO+H2O��CD�ε��¶ȳ�����200�棬����̼��ͭ�ķֽ��¶ȣ��ʴ�Ϊ��CuCO3
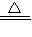 CuO+CO2����
CuO+CO2������6��AB����������ͭ�ڷֽ⣬������������3.6�ˣ�CD����̼��ͭ�ڷֽ⣬������������4.4�ˣ�
Cu��OH��2
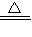 CuO+H2O CuCO3
CuO+H2O CuCO3 CuO+CO2��
CuO+CO2���ӷ���ʽ���Կ���һ��������ͭ�ֽ������һ��ˮ��һ��̼��ͭ�ֽ�����һ��������̼����AB�ε�������ͭ������3.6��ˮ��������0.2��ˮ����Ҫ0.2��������ͭ��CD�ε�̼��ͭ�ֽ������4.4�˶�����̼���൱�ڼ�����0.1��������̼����Ҫ0.1��̼��ͭ������a��b=2��1���ʴ�Ϊ��a��b=2��1��
��7������ͭ���Ⱥ������ֻ���٣�Ӧ��������ͭ�е���Ԫ��ת��Ϊ������24g����ͭ����Ԫ�ص�����=24g×
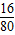 =4.8g������2.4g��˵��������ͭ�еIJ�����Ԫ��ת��Ϊ������
=4.8g������2.4g��˵��������ͭ�еIJ�����Ԫ��ת��Ϊ�������ʴ�Ϊ��4CuO
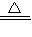 2Cu2O+O2����
2Cu2O+O2�����������ڽ������ʱ������Ҫ�����е�֪ʶ��֪��Ȼ����ѧ����֪ʶ���н�𣬴������ѶȽϴ�Ҫϸ�Ľ��з������

��ϰ��ϵ�д�
�����Ŀ