��Ŀ����
����Ŀ��ij����С���һ�������ˮ���ŷŵķ�Һ�к���CuSO4��H2SO4��
(1)CuSO4���ؽ����Σ����뵰���ʷ���______�仯(ѡ���������ѧ��)��ʹ������ʧȥԭ�����������ܣ�����CuSO4�ж���
(2)Ϊ�˲ⶨ��Һ��CuSO4��������������С��ȡ100g��Һ����μ���NaOH��Һ���������������Cu(OH)2����������������NaOH��Һ��������ϵ��ͼ��ʾ��

��ʵ����������Cu(OH)2������������_____g��ͼ��OA��δ����Cu(OH)2������ԭ��:______��
�ڼ����Һ��CuSO4����������(�����ȷ��0.1%)��_____����֪��CuSO4+2NaOH=Cu(OH)2��+Na2SO4��
������ȡl00g��Һ����������μ��������BaCl2��Һ��ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�������Ƶ�����Ϊmg����_____ (��ܡ����ܡ�)�ú�m�Ĵ���ʽ�����Һ��CuSO4������������
���𰸡���ѧ 3.92 ������ͭ������ˮ�������ܹ����ᷴӦ���ɿ��ܵ��Ȼ�ͭ������OA�����������ƺ����ᷴӦ������������ͭ��Ӧ 6.4% ����
��������
��1��CuSO4���ؽ����Σ����뵰���ʷ�����ѧ�仯��ʹ������ʧȥԭ�����������ܣ�����CuSO4�ж���
��2������ͼ��֪ʵ����������Cu��OH��2������������ 3.92 g��ͼ��OA��δ����Cu��OH��2������ԭ��������ͭ������ˮ�������ܹ����ᷴӦ���ɿ��ܵ��Ȼ�ͭ������OA�����������ƺ����ᷴӦ������������ͭ��Ӧ��
���裺��Һ��CuSO4����������Ϊx��
![]()
![]()
x��6.4%
���������������ͭ���ܺ��Ȼ�����Ӧ���ɳ��������Բ��������ᱵ�����������Һ��CuSO4������������

����Ŀ����������װ��ͼ���ش��й����⣺

��1��ʵ��������ȡ����ʱ������_____����Ӧ����ȷ�����巢��װ�á�����±���
���� | ���巢��װ�� | ��ѧ����ʽ |
���� | A | _____�� |
������̼ | B | _____�� |
��2��ʮ�����ͣ���仯ѧ����������ͼװ���Ʊ��������������װ���������Ũ����Ļ������ڻ�¯�ϼ��ȣ������������ö���������ɵĿڴ��ռ�����������ͼ�еķ���װ�ý��и�ʵ�飬����ʵ���_____������ĸ����

��3��ʵ��������п����ϡ���ᷴӦ��ȡ��������װ��B��Ϊ����װ�ã�����©�����¶˱���_____����װ��D��Ϊ����װ�ã��÷�Һ©������ϡ������ŵ���_____����Һ©��������_____�滻��
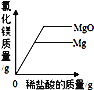
��4��һ��������п����98g��������Ϊ10%��ϡ����ǡ����ȫ��Ӧ��������������������Ƕ���_____�����������Һ���������������Ƕ���?_____��д�����������
����Ŀ��NH4Cl��Na2SO4���ܽ�ȱ����ܽ���������¡�����˵����ȷ���ǣ�������
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��S/g | NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 60.2 |
Na2SO4 | 9.6 | 20.2 | 40.8 | 48.4 | 47.5 | 47.0 | |

A. t3Ӧ����40�桫50��
B. ��t3��ʱNa2SO4�ı�����Һ������t1�棬c�㽫������c��b��a�˶�
C. �ס��ұ�����Һ��t2���µ�t3�����ʵ���������������
D. ��d��ס�����Һ����c�㣬�ɲ��õķ���ֻ�м������ʼס��ң�������������������