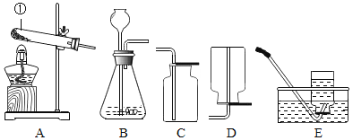
��Ŀ����
����Ŀ��̼���仯������������������ᷢչ������ء�
(1)Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�_____��
(2)����Ŀǰ�����ĵ�������Ҫ���Ի�ʯ��Դ����ʯ��Դ�Ĵ���ʹ�û����SO2��CO��NO2��CO2������ͷ۳��������ڴ����еĺ���������������������ЧӦ��ǿ��ȫ�������ů����___�����γ��������Ҫ��____��
(3)�۲���ͼ��ʾ��ʵ�顣��ͼ��������������סȼ�ŵ����۲쵽�������ǣ�һ����ߵ�������Ϩ�𣬵͵������Ϩ��ͬʱ���۲쵽�������ڱڱ�ڡ���ʵ��������ʾ���ǣ���¥���Ż�ʱ��ȷ�������ķ����� ____(����ĸ���)��
�ٴ������� �ڳ�վ�������ܳ� ����ʪë����ס���� ����ǽ�Ƿ�������Ѹ����������
A��ֻ�Т� B. �ں͢� C. �ۺ͢� D.�����ں͢�
(4)Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ������ʾ��
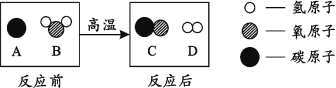
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ________��
�ڸ�������ʾ��ͼ�����ܵõ���Щ��ѧ�仯����Ϣ?(��дһ��)___��
(5)Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϡ����о�����NH3ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ������NH3ȼ�շ�Ӧ�Ļ�ѧ����ʽ����������4NH3 +3O2![]() 6H2O + ____��
6H2O + ____��
���𰸡���Ȼ�� CO2 SO2(��NO2) C C+H2O ![]() CO + H2 ��ѧ�仯������ԭ�ӵ����ࡢ��Ŀ����(���������𰸼���) 2N2
CO + H2 ��ѧ�仯������ԭ�ӵ����ࡢ��Ŀ����(���������𰸼���) 2N2
��������
��1�������Ļ�ʯȼ����ú��ʯ�͡���Ȼ����
��2����ʯȼ�ϵ�ȼ�ղ����У�������̼�������γ�����ЧӦ��������������������������ˮ�γ����ꡣ
��3��¥���Ż�ʱ�������¶Ƚϸߣ�ʹ������̼��������ܶȱ�С�������ƶ�������Ա����ʱ����ʪë����ס���ӣ���ǽ�Ƿ�������Ѹ��������ѡC��
��4��������ʾ��ͼ��֪���÷�Ӧ�ķ�Ӧ����̼��ˮ����������һ����̼�����������ݻ�ѧ����ʽ����д����Ӧ����ʽΪ��C+H2O ![]() CO + H2���ڸ���ͼʾ��֪����ѧ�仯������ԭ�ӵ����ࡢ��Ŀ������
CO + H2���ڸ���ͼʾ��֪����ѧ�仯������ԭ�ӵ����ࡢ��Ŀ������
��5����Ӧ4NH3 +3O2![]() 6H2O + ____�У���Ӧ���е����⡢����ԭ�Ӹ����ֱ���4��12��6���������е����⡢����ԭ�Ӹ����ֱ���0��12��6����δ֪���й���4����ԭ�ӣ���Ϊ2N2
6H2O + ____�У���Ӧ���е����⡢����ԭ�Ӹ����ֱ���4��12��6���������е����⡢����ԭ�Ӹ����ֱ���0��12��6����δ֪���й���4����ԭ�ӣ���Ϊ2N2

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
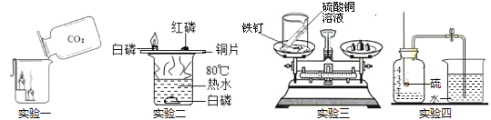
������ҵ��ٳɳ����½������������ϵ�д�����Ŀ��ij��ѧС��ͬѧ��þ���ڿ�����ȼ��ʵ��ʱ�������������л���������ɫ���壬�����һ����������⣬����չ��������̽��������ϸ�Ķ����������ǵ�̽��֮�á�
��̽��Ŀ�ģ�̽����ɫ����ʱþ�������ʲô���ʷ�Ӧ�����ģ�
���������ϣ�
��1��ϡ������һ�㲻���������ʷ�Ӧ��
��2��������þ����ˮ��Ӧ����������ɰ�ɫ���塣
���������룩���������и��ɷ֣����в��룺
��1������������� ������ǵ��� �������_____________��
��ʵ��һ��þ���������ķ�Ӧ
��һ����ͬѧ̽����Ӱ�����������ȡ�����ٶȵ�ij�����أ�����������ʵ�鲢��¼�˸����ռ���ͬ�����������Ҫ��ʵ�����±���
ʵ�� | ���������Ũ�� | ����������Һ��������g�� | �������̵�������g�� | �ռ�ʱ�䣨s�� |
1 | 10% | 20 | 1 | 40 |
2 | 15% | 20 | 1 | 20 |
3 | 20% | X | 1 | 12 |
��2����3��ʵ���У�X��ֵӦΪ_______________��
��3��ʵ����ۣ�����ͬ�����£�___________����������ֽ��Խ�졣
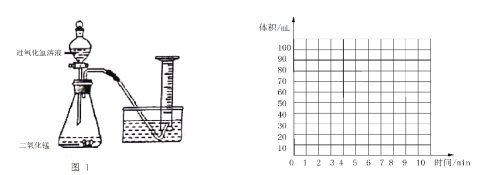
��������ͬѧ����Ͳ��ʢ��ˮ��������ˮ���У�ͨ����Һ©����20 mLijŨ�ȵù���������Һ������ƿ�У���¼��Ͳ������������ͼ1�����������±���
ʱ��/min | 1 | 3 | 5 | 7 | 9 | 10 |
��Ͳ����/mL | 60.0 | 82.0 | 88 | 89.5 | 90 | 90 |
��4����������Ϲ��̺�ʵ�������ۺϷ������������������������_________mL��
��5���������������л��Ƴ�0��10min�������������ʱ��仯������____________��
������ȼ��þ�����۲�����
��þ����ĥ��������ȼ������ʢ�������ļ���ƿ�У������������̣����ɰ�ɫ���塣
ʵ����ۣ�þ��������Ӧ���ɰ�ɫ������þ��
��ʵ�����þ���뵪���ķ�Ӧ
��һ���ռ������ĵ���
������ȼ��þ�����۲�����
��þ����ĥ��������ȼ������ʢ�е����ļ���ƿ�У�ƿ�ڱڸ���һ�㵭��ɫ�Ĺ��塣
��6��ʵ����ۣ�þ�뵪���ڵ�ȼ�õ������·������Ϸ�Ӧ���ɵ���ɫ�ĵ���þ��Mg3N2�����÷�Ӧ�Ļ�ѧ����ʽ��______________��
��̽������1��
��7����������������þ�ڿ�����ȼ�ղ�����ɫ����IJ����Dz���________������ţ�������
��̽������2��
��8�������������þ��������п������ʵ�飬ʵ�������������������ʵ��ֵ_______���ƫ��ƫС������
��ʵ��عˣ�ʵ���ҳ��ú���ȼ�յķ����ⶨ����������������ͼ2�������ں�����Ҫ��ƿ���ȼ������ƿ�ڣ�����������Ⱦ�Լ���
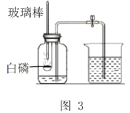
��ʵ��Ľ���ͬѧ��ѡ�����Ż����͵İ��ף�����װ��ͼ���˸Ľ���ͼ3��.��Ҫ�����ǣ���ʵ���ݻ�Ϊ180mL�ļ���ƿ���װ��50 mL��ˮ���ٰ�ͼ���Ӻ������������ȵIJ�������������������ȼ��
��9�����״�ȼ�յ�Ϩ����ȴ�Ĺ����У�ƿ��ˮ��ı仯��_______________��
��10����ʵ�����������ռ���ƿ��ˮ�����ԼΪ_________mL��
��11������ƿ��Ԥ��װ��50 mLˮ�����ã���һ��Ϊ�˼ӿ켯��ƿ����ȴ�ٶȣ��������Ҫ��_____��
��ʵ���뽻����
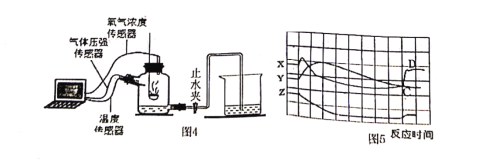
��12��Ϊ�˰���ͬѧ�Ǹ��õ�������������������ⶨ��ʵ��ԭ������ʦ���ô�����������ʱ������ʵ��װ�ã�ͼ4���ڵ�ѹǿ���¶Ⱥ�����Ũ�ȣ��������߱仯������ͼ5��ʾ��Y���߱�ʾ����________����¶ȡ���������Ũ�ȡ���ѹǿ�����仯���ƣ�X������CD��������ԭ����________��