��Ŀ����
25���±����Ȼ��ƺ�������ڲ�ͬ�¶�ʱ���ܽ�ȣ����ݴ˱��ش�
��1��40��ʱ���Ȼ��Ƶ��ܽ��Ϊ
��2�������Ȼ��Ƶı�����Һ�еõ��Ȼ��ƾ��壬ͨ������
��3��60��ʱ��105g����ر�����Һ�����ʡ��ܼ�������֮��Ϊ
��4��30��ʱ����40g����ع������ʢ��50gˮ���ձ��У����ٽ��ձ����������µ�50�棨������ˮ������������Һ�б仯����
A���ܼ������� B����Һ������ C�����ʵ��������� D�����϶����䣮
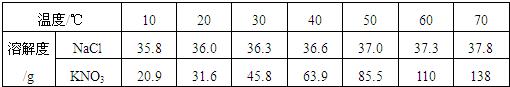
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | |
| �ܽ�� ��g/100gˮ�� |
NaCl | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
| KNO3 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
36.6
g/100ˮ����2�������Ȼ��Ƶı�����Һ�еõ��Ȼ��ƾ��壬ͨ������
�����ܼ�
��������3��60��ʱ��105g����ر�����Һ�����ʡ��ܼ�������֮��Ϊ
11��10��110��100��55��50��
����4��30��ʱ����40g����ع������ʢ��50gˮ���ձ��У����ٽ��ձ����������µ�50�棨������ˮ������������Һ�б仯����
B��C
��ѡ��A��B��C��D����A���ܼ������� B����Һ������ C�����ʵ��������� D�����϶����䣮
��������1���ɱ������ݶ������ɣ�
��2�������Ȼ��Ƶ��ܽ�����¶ȱ仯�ܲ����ԣ���ֻ�ܲ��������ܼ��ķ��������ʽᾧ������
��3��������ͬ�¶��±�����Һ���������ܼ��ı�ֵ��Ƚ����
��4������������ܽ�����¶ȵ����߶����߽����
��2�������Ȼ��Ƶ��ܽ�����¶ȱ仯�ܲ����ԣ���ֻ�ܲ��������ܼ��ķ��������ʽᾧ������
��3��������ͬ�¶��±�����Һ���������ܼ��ı�ֵ��Ƚ����
��4������������ܽ�����¶ȵ����߶����߽����
����⣺��1���ɱ������ݿ�֪���ҵ�40��ʱ�����ܽ�ȣ�
�ʴ�Ϊ��36.6
��2�������Ȼ��Ƶ��ܽ�����¶ȱ仯�ܲ����ԣ���ֻ�ܲ��������ܼ��ķ��������ʽᾧ������
�ʴ�Ϊ�������ܼ�
��3����ͬ�¶��±�����Һ���������ܼ��ı�ֵ��ȣ�60��ʱ��105g����ر�����Һ�����ʡ��ܼ�������֮��Ϊ110��100=11��10��
�ʴ�Ϊ��11��10
��4��������ܽ�����¶ȵ����߶����ߣ�������º�ԭ��δ�ܵ������������ܽ���һ���֣��������ʵ�������������ˣ���Һ������Ҳ��֮���
�ʴ�Ϊ��B��C
�ʴ�Ϊ��36.6
��2�������Ȼ��Ƶ��ܽ�����¶ȱ仯�ܲ����ԣ���ֻ�ܲ��������ܼ��ķ��������ʽᾧ������
�ʴ�Ϊ�������ܼ�
��3����ͬ�¶��±�����Һ���������ܼ��ı�ֵ��ȣ�60��ʱ��105g����ر�����Һ�����ʡ��ܼ�������֮��Ϊ110��100=11��10��
�ʴ�Ϊ��11��10
��4��������ܽ�����¶ȵ����߶����ߣ�������º�ԭ��δ�ܵ������������ܽ���һ���֣��������ʵ�������������ˣ���Һ������Ҳ��֮���
�ʴ�Ϊ��B��C
�����������Ƕ������ܽ�ȵĿ��飬������ص����������ܽ�ȵĸ�����������ܽ�ȷ�����������⣬��������֪ʶ�����⣮

��ϰ��ϵ�д�
�����Ŀ
�±����Ȼ��ƺ�������ڲ�ͬ�¶��µ��ܽ��S����/100��ˮ�������ݴ˱��ش�
��1��50��ʱ������ص��ܽ��Ϊ85.5��/100��ˮ����ʾ�ĺ����� ��
��2����60��ʱ��10��ˮ�м���12������أ���ֽ����������Һ������Ϊ �ˣ�����Һ��������������Ϊ ������������ȷ��0.1%��
��3���ɱ������ݿ�֪������غ��Ȼ�����ij�¶ȷ�Χ���ܽ����ȣ�����¶ȷ�Χ�� ��
��4����ˮ��ʳ�����������ܼ��ķ�������ԭ���� ��
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
| NaCl���ܽ�� | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 |
| KNO3���ܽ�� | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 | 138.0 |
��2����60��ʱ��10��ˮ�м���12������أ���ֽ����������Һ������Ϊ
��3���ɱ������ݿ�֪������غ��Ȼ�����ij�¶ȷ�Χ���ܽ����ȣ�����¶ȷ�Χ��
��4����ˮ��ʳ�����������ܼ��ķ�������ԭ����
 ��2013?�ֿ���һģ���±����Ȼ��ƺ�������ڲ�ͬ�¶�ʱ���ܽ��
��2013?�ֿ���һģ���±����Ȼ��ƺ�������ڲ�ͬ�¶�ʱ���ܽ��