��Ŀ����
С��ͬѧ���ڿ����з���һ��ʱ��ġ�ͭ��������˿����������ͭ��Һ�Ƴ�����ͼ���ĵijɷ�ʱ�з�����ȡ��64.1�˹�����Ʒ����10%���������ܽ⣬��Һ����ɫ�������������ʣ�����������10%�����������仯��ϵ��������ͼ��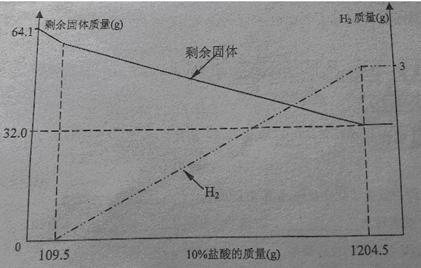
���ͼ�����ݷ�����
��1����ͼ��֪��CuԪ�ص�����_____g��64.1g��ͭ������Cu��Al��_____���ѧʽ��
��2�����ϻ�ѧ����ʽ����á�ͭ������AlԪ�ص�����������
��3��ֻ֪����ͭ��������m1������10%��������������m2��,Ҳ�������ͭ��������Ԫ�����������������ʽΪ_______________(��m1��m2��ʾ���ɲ�����)
��1��32.0 Al2O3 (2)64.3% ��3��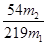 ��100%
��100%
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ʹ�õ�ˮ���ײ�������һ��ˮ��������Ҫ�ɷ���̼��ƺ�������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±���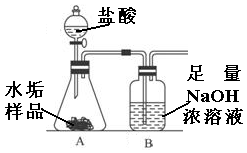
| | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| Bװ�����ӵ�������g�� | 2.17 | 2.22 | 2.21 | |
��������ʵ�������ݺش�
�ŵ�һ��ʵ�������ݽϵ͵�ԭ���� ��
��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ �ˣ�
��ˮ����Ʒ��������þ��ƽ����������Ϊ ������������ȷ��0.1��
ij�����������Ĵ����к�������NaCl���ʣ����Ʒ��װ���ϱ��У�̼���ơ�96%��Ϊ��֤ʵ�ò�Ʒ��̼���Ƶĺ�����ijͬѧȡ12g����Ʒ�����ձ��У��Ƶ��ձ�����Ʒ������Ϊ132.0g�ٰ�100gϡ����ƽ���ֳ�4�μ����ձ��У�ÿ�γ�ַ�Ӧ���ձ���ʣ�������������£���ÿ�η�Ӧ������CO2���嶼ȫ�����ձ����ݳ���
| ����ϡ������� | 1 | 2 | 3 | 4 |
| ����ϡ��������/g | 25 | 25 | 25 | 25 |
| ��ַ�Ӧ���ձ���ʣ����������/g | 155.2 | 178.4 | 202.6 | 227.6 |
������ݱ������ݷ������ٵ�1�γ�ַ�Ӧ�������CO2������������ ��g
�ڸò�Ʒ��̼���Ƶ����������Ƕ��٣��ò�Ʒ�Ƿ�ϸ������ش𣬼�������ȷ��0.1%��
���ʶ����ũ����IJ���������Ҫ�����ã�̼泥�̼����泥�����ũ�����Ѿ���ʹ�õ�һ�ֵ��ʣ�̼�������ˮ���ܳ�ʱ���·ֽ⣬�¶�Խ�߷ֽ�Խ�죬����ʱ�ų�������ijУ��ѧ��ȤС��Ϊ�ⶨij̼立�����Ʒ�Ĵ��ȣ���8.5g��Ʒ����������Ũ����������Һ�й��ȣ���̼��е����ʲ����������Ʒ�Ӧ����Ӧ�ķ���ʽΪNH4HCO3+2NaOH = Na2CO3+2H2O+NH3�������Ѳ���������NH3��������������Һ���գ�ͬʱ������������������Һ���ӵ�������������±���ʾ��
| ʱ��/S | 0 | 20 | 30 | 40 | 60 | 80 | 100 | 120 |
| ��������/g | 0 | 0.7 | m | 1.2 | 1.5 | 1.6 | 1.7 | 1.7 |
�Իش��������⣺
��1������̼����淋����ʣ�����Ϊ��ʹ��̼立���ʱӦע���������___________________��
��2����������ֽ�ϣ��Է�Ӧʱ��Ϊ�����꣬�Բ���NH3����Ϊ�����꣬�������������������ʱ��仯�Ĺ�ϵ���ߣ����жϱ���mԼΪ_________________��
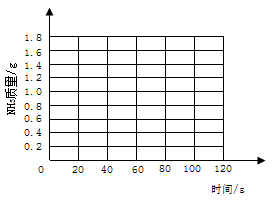
��3���Լ������Ʒ��̼����淋�����������д��������̣�����������һλС������
������ܽ�û�ձ�����ձ���ȡ���ܴﵽ�濪���������ͣ�������������ȡ��ʹ�ø�װ�õ���
| A���ô���ʯ��ĩ��ϡ���ᷴӦ��ȡ������̼ |
| B����п����ϡ���ᷴӦ��ȡ���� |
| C���ö������̷�ĩ��˫��ˮ��Һ������ |
| D���ÿ�״����ʯ��ϡ���ᷴӦ��ȡ������̼ |
�ռ�ij������ķ�������Ҫȡ����������������ʡ�ij�����������ˮ���ռ��������������ſ������ռ������������е�����Ϊ
| A��������ˮ���ܶȱȿ���С |
| B�����ܻ�������ˮ���ܶȱȿ����� |
| C�����ܻ�������ˮ���ܶȱȿ���С |
| D��������ˮ���ܶȱȿ����� |