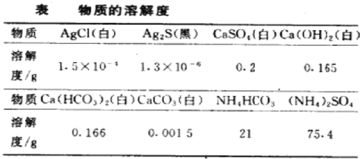
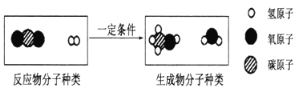
��Ŀ����
����Ŀ��ijʯ�ͻ����������ķ�ˮ�к������������������ͭ������ʵ������15%������������Һ����������ˮ��Ʒ����������������Һ�����������������ϵ��ͼ��ʾ��
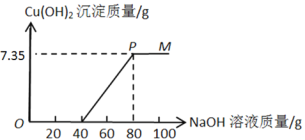
(1)��ͼʾ�����μ�����������Һ�Ĺ��������ȷ�����Ӧ�Ļ�ѧ����ʽ��_______��
(2)��P��ʱ��������pHֵ_____(��������������С���������)7��
(3)��P��ʱֹͣ��������������Һ�����˺���Һ�������պ�Ϊ200 g������Һ��Na2SO4����������Ϊ__________��
���𰸡� H2SO4+2NaOH�TNa2SO4+2H2O ���� 10.65%
�������������������ͭ�Ļ��Һ�м�����������ʱ�����������������Ʒ�Ӧ���������ƺ�ˮ��������ȫ��Ӧ��������ͭ�ٺ��������Ʒ�Ӧ����������ͭ��������������(1)��ͼʾ�����μ�����������Һ�Ĺ��������ȷ�����Ӧ���������������Ƶķ�Ӧ����ѧ����ʽ��H2SO4+2NaOH�TNa2SO4+2H2O��(2)��P��ʱ������ͭ�����ᡢ�������ƶ���ȫ��Ӧ����Һ�е�����ֻ�������ƣ�������pHֵ����7��(3)��P��ʱֹͣ��������������Һ�����˺���Һ�������պ�Ϊ200 g����P��ʱ��Һ��Na2SO4������Ϊx��H2SO4+2NaOH�TNa2SO4+2H2O��2NaOH+CuSO4= Cu(OH)2��+Na2SO4������������ʽ��֪�������ƺ������ƵĹ�ϵʽΪ��
2NaOH ~ Na2SO4
80 142
80g��15% x
80/80g��15%=142/x x=21.3g, ��Һ��Na2SO4����������Ϊ21.3g��200g��100%=10.65%��
����Һ��Na2SO4����������Ϊ10.65%��

����Ŀ�����л�ѧ���������ȷ���Ҽ��к�����壬�������������
ѡ�� | �� �� | ��ѧ���� |
A | �ؿ��к������Ľ���Ԫ�� | AL |
B | �����ǵĻ�ѧʽ | C6H12O6 |
C | ����������Һ�Ͷ������̻�������� | H2O2 |
D | �����ӵķ��� | Cl1- |
A. A B. B C. C D. D