��Ŀ����
����Ŀ����ѧ��Դ����������������ش����������е��й����⣺
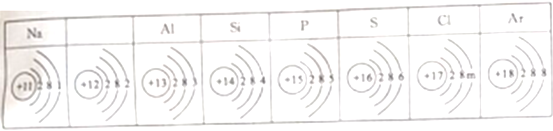
��1���ӻ�ѧ�뽡���Ƕȣ�
�� ��������ȱ��ijЩԪ��ʱ��Ӱ�콡��������ȱ��������__________��
�� ������������Σ�����彡������__________������ĸ��ţ���
A����̼�����Ʊ��Ƹ�� B�����̷������ӻ���ԭ�������谷
C���ù�ҵʯ���ҽ�Ʒ���ʯ���������� D����ù��Ļ���ե������ʳ��
��2���ӻ�ѧ����Դ�Ƕȣ�
�� ��Ȼ���������ճ����������г��õĻ�ʯȼ��֮һ��
��Ȼ������____�������������������������Դ����Ȼ����ȫȼ�յĻ�ѧ����ʽΪ___��
�� ��֪��������Դ����Щ��_____________����дһ�֣�
��3���ӻ�ѧ����ϽǶȣ�
�� �·������е���ë����______�����Ȼ��ά���ϳ���ά������
�� �����ֶ������Ͻ����ǵ����ܲ�ͬ��ԭ����__________________________��
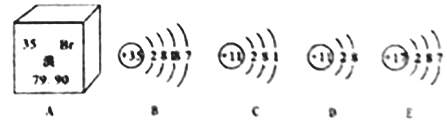
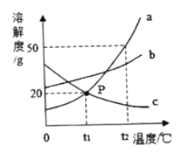
�� �Ͻ�ĺܶ�������������ǵĴ�������ͬ����ͼ���ܱ�ʾ��Ǧ�Ͻ����_______������š�a������b����c������

�� þ�Ͻ���Ϊ��21������ɫ�����ṹ���ϡ���Mg17Al12��һ�������þ�Ͻ�ͨ��ѡ������������ǿ�����������ԭ����_________________��дһ����ѧ����ʽ�����úϽ���һ�ִ�����ϣ���ȫ�����õ�MgH2��Al�� ��������̡�����__________�����������ѧ�����仯��
��4���ӻ�ѧ�뻷���Ƕȣ�
�̵����к��д���CO2���������������������״���CH3OH���Ȳ�Ʒ��
�١�����CO2���ڸ�ѹʱ���̵����е�CO2�ܽ��ڼ״����õ�CO2�ļ״���Һ��������Һ��������___________��
�� �á�������CO2�����״�����Ӧ����ʾ��ͼ���£�
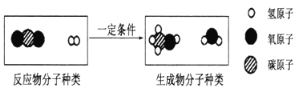
�÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
���𰸡� ƶѪ A �������� CH4+2O2��ȼ2H2O+CO2 ̫����(��������) ��Ȼ��ά ��̼����ͬ a 2Mg+O2=2MgO����4Al+3O2=2Al2O3�� ��ѧ CO2 CO2+3H2![]() CH3OH+H2O
CH3OH+H2O
����������1�������������������ܽ�������������ʵ����ʷ����������2���ٸ�����Ȼ�����γɼ���Ҫ�ɷּ����������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ��𣻢ڸ���̫���ܡ����ܵ���������Դ�����3���ٸ�����ë������Ȼ��ά��𣻢ڸ���̼������ͬ��𣻢۸��ݺϽ���۵��������ǵĴ��������۵�ͽ�𣻢ܸ���þ�������ڿ����е�������Ӧ��𣻸��������������ɽ�𣻣�4���ٸ��ݶ�����̼�ܽ��ڼ״���𣻢ڸ��ݷ�Ӧ����ʾ��ͼ�����1��������ȱ��������ƶѪ����A��̼�������׳�С�մ��Ƿ��ͷ۵���Ҫ�ɷ֣����������Ƹ������ȷ��B���������������谷�������ֳ������ϵͳ�������ס�������ʯ�����ɽ�һ���շ����װ������� C����ҵʯ���ҽ�Ʒ���ʯ����ж����ʻ������Ľ�������Ӱ�죬����D��ù��Ļ����к��л���ù�أ����ж�������ʳ�ã�����ѡA����2������Ȼ���ǻ�ʯȼ�ϣ����ڲ���������Դ�������������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2��ȼ2H2O+CO2����̫������������Դ����3������ë������Ȼ��ά���� �����ֶ������Ͻ����ǵ����ܲ�ͬ��ԭ���Ǻ�̼����ͬ���� �Ͻ�ĺܶ�������������ǵĴ�������ͬ���Ͻ���۵��������ǵĴ��������۵�͡���ͼ���ܱ�ʾ��Ǧ�Ͻ����a����þ�����������ڿ����е�������Ӧ��������þ����������������Ӧ�Ļ�ѧ����ʽΪ2Mg+O2=2MgO����4Al+3O2=2Al2O3������������̡������������ɣ����ڻ�ѧ�仯����4���ٶ�����̼�ܽ��ڼ״����γɶ�����̼�ļ״���Һ���������Ƕ�����̼�����ɷ�Ӧ����ʾ��ͼ��֪���÷�Ӧ�Ƕ�����̼��������һ�������·�Ӧ���ɼ״���ˮ����Ӧ�Ļ�ѧ����ʽΪCO2+3H2![]() CH3OH+H2O��
CH3OH+H2O��

����Ŀ��ijʵ��С�����÷�����Һ�Ʊ�K2SO4���о�CaSO42H2O���ȷֽ�IJ��
��һ��K2SO4���Ʊ�
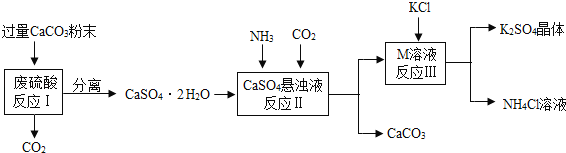
��1����CaCO3�гɷ�ĩ��Ŀ����___________________________________��
��2��M���ʵĻ�ѧʽΪ__________��
��3����Ӧ����������ʵ��ܽ�����±���
���� | KCl | K2SO4 | NH4Cl | M |
�ܽ��/g��25�棩 | 34.0 | 11.1 | 37.2 | 19.5 |
��Ӧ���ڳ�������ʵ�ֵ�ԭ����_______________________________________��
��4�����������п�ѭ��ʹ�õ�������CO2��_____________����д��ѧʽ����
�������о�CaSO42H2O���ȷֽ�IJ���
��5���������CaSO42H2O�г�����CaCO3�����������ȥCaCO3��
�÷�Ӧ�Ļ�ѧ����ʽ_______________________________________________��
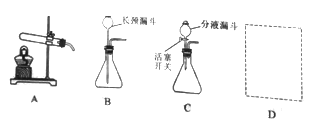
��6��Ϊ�˲ⶨCaSO42H2O��CaCO3��������x��y��ʵ��С��������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�顣ע����ʯ�ҵ���Ҫ�ɷ�ΪNaOH��CaO��
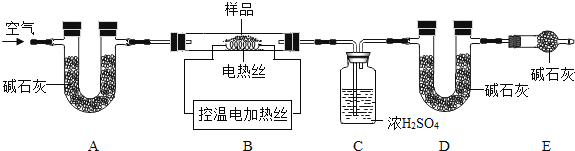
�� ʵ��ǰ����Ҫ________________����װ����Ʒ��װ��A��������_________________��
�� ��֪��CaSO42H2O��160������CaSO4��1350��ʱCaSO4��ʼ�ֽ⣻ CaCO3��900��ʱ�ֽ���ȫ��
�ֿ���Bװ���¶�900�����ʵ�鲢�ɼ����������ݣ�
a����Ӧǰ����������Ʒ������m1g b����Ӧ�������й��������Ϊm2g
c��װ��Cʵ�������m3g d��װ��Dʵ�������m4g
ijͬѧѡ��b��d��c��d����������x��y��ֵ������װ��E����ʵ��ⶨ�����______�����ƫ����ƫС������Ӱ�족������Ϊ����ѡ��������________________��ѡ����ţ������������Ҳ�����x��y��ֵ��
��7��CaSO42H2O���Ȼ���ʧȥ�ᾧˮ��ȡ����CaSO42H2O����3.44g�����ڣ�5����ʵ��װ��B�н��м��ȣ��ⶨ�����������¶ȵı仯�����ͼ��ʾ��
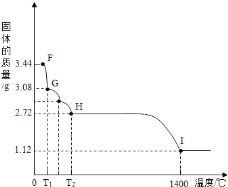
��G�����Ļ�ѧʽ��_________________��