��Ŀ����
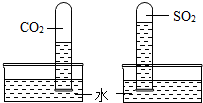
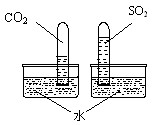
��12�֣���1��ѧϰ������̼����ʱ����ʦ��������ͼ��ʾ��ʵ�顣������֣�����ʯ��ˮ����ǡ���д������ʯ��ˮ������йط�Ӧ�Ļ�ѧ����ʽ_____________________��

��ʦ�����������������������ͬ���ڼ��������������ƵĻ�ѧ�������ƣ���ô�����������ܷ��������̼��Ӧ�أ�
ͬѧ��Χ����������⣬ͨ���������ϵ�֪�������£�CO2+2NaOH=Na2CO3+H2O��
���ţ�����ʦָ���£����������ʵ�飺������ʵ���еij���ʯ��ˮ��������������Һ��������ͨ��CO2���۲쵽��ʵ������Ϊ_________________________��
��2��Ϊ����֤CO2�����������Ʒ�����ѧ��Ӧ��ͬѧ�����������ʵ��

�ټ�ʵ�飺��ʢ��CO2�����Ͽ�Ȫˮƿ���������м���һ����NaOH��Һ��Ѹ������ƿ����������ֹһ�����۲쵽��ʵ������Ϊ________________________��
����ʵ�飺��ʢ��CO2�ļ���ƿ�е���һ����NaOH��Һ��Ѹ����ƿ�ڴ�����һ��ȥ���켦������ֹһ�����ɹ۲쵽��ʵ������Ϊ________________________��
��3����������ʵ�����ͬѧ������ɣ���Ϊ����ʵ�鲻����˵��CO2���������Ʒ����˻�ѧ��Ӧ������Ϊ�ⲿ��ͬѧ����������ɣ���Ҫ���ǵĸ���������________________��Ϊ�˽�һ����֤CO2�����������Ʒ�����ѧ��Ӧ��ͬѧ�������������ʵ�飺

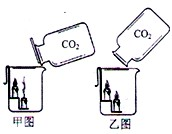
��Aƿʢװ���Լ���________________��Bƿʢװ���Լ���______________��ʵ��ʱӦ_____
[����رա�]����K��
�ڽ��д�ʵ��ʱ��ͨ��CO2�����Ӧ__________[����ڡ��������ڡ���С�ڡ�]Aƿ��Һ�������
����֤��CO2�����������Ʒ�����ѧ��Ӧ��ʵ��������______________________________��
��12�֣�
��1��CO2+Ca(OH)2=CaCO3��+H2O�������Ա仯��
��2��������ƿ��� �ڼ���������ƿ��
��3��������CO2����ˮ�������ƿ���
��NaOH��Һ������ʯ��ˮ���رա�
�ڴ���
��A��B�����������
����:��

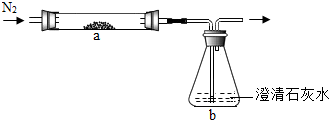
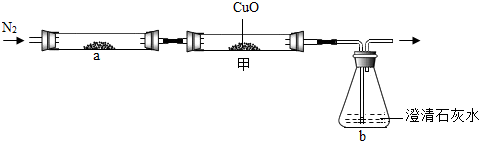
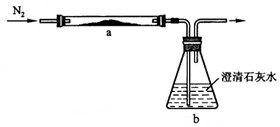
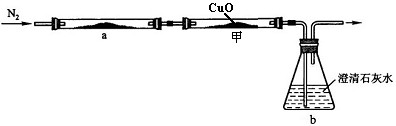
 ��2013?���ݣ�С��ѧϰ������̼֪ʶ�����˶�����̼������ˮ���ܽ��Ե�̽������ʵ�鲽�輰װ�����£�
��2013?���ݣ�С��ѧϰ������̼֪ʶ�����˶�����̼������ˮ���ܽ��Ե�̽������ʵ�鲽�輰װ�����£�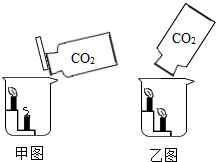 ��ѧ��ȤС���ͬѧ��̼��̼�������������һϵ����չ��̽��ѧϰ��
��ѧ��ȤС���ͬѧ��̼��̼�������������һϵ����չ��̽��ѧϰ��