��Ŀ����
����Ŀ��̼Ԫ��������������ʵĻ���Ԫ�أ��ش����к�̼Ԫ�ص��й�����(7��)
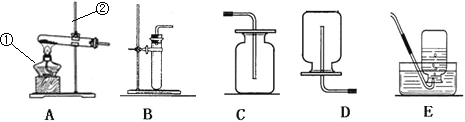
(1)��������̼�������ҪΪ�˼���_____________���ŷ�����
(2)���������̼�����ó���ʯ��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ____________________��
(3)��Ȼ��(��Ҫ�ɷ���CH4)����ȼ��_____________________________(�û�ѧ����ʽ��ʾ)��
(4)Ϊ������������CO2���ŷţ���ѧ�ҽ�CO2��H2�ڴ����ͼ��������·�Ӧ,ת��Ϊˮ�ͼ��飬�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________������˵:�������ж�����̼�ĺ���Խ��Խ���������Ƿ�ͬ������˵��(����������������)________������____________________��
(5)���ʯ��ʯī���������ʲ���ϴ���Ҫԭ����_______________________��
���𰸡� CO2 CO2+Ca(OH)2=CaCO3��+H2O CH4+2O2=CO2+2H2O CO2+4H2 2H2O+CH4 �� ��������CO2����̫�ͣ���Ӱ��ֲ��Ĺ������ ̼ԭ�ӵ����з�ʽ��ͬ
2H2O+CH4 �� ��������CO2����̫�ͣ���Ӱ��ֲ��Ĺ������ ̼ԭ�ӵ����з�ʽ��ͬ
��������(1)����̼��������������������ģ���Ҫ��Ϊ�˼��ٶ�����̼���ŷ���(2)������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca(OH)2=CaCO3��+H2O ��(3)�����������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ����ѧ����ʽ��ʾΪ��CH4+2O2![]() CO2+2H2O��(4)������̼�������ڴ����ͼ��ȵ������·�Ӧ����ˮ�ͼ��飬��ѧ����ʽΪ��CO2+4H2
CO2+2H2O��(4)������̼�������ڴ����ͼ��ȵ������·�Ӧ����ˮ�ͼ��飬��ѧ����ʽΪ��CO2+4H2![]() 2H2O+CH4������˵����������CO2�ĺ���Խ��Խ�á�����ͬ������˵���������ǣ���������ж�����̼�ĺ���̫�ͣ���Ӱ��ֲ��Ĺ�����ã�(5)���ʯ��ʯī���������ʲ���ϴ���Ҫԭ����̼ԭ�ӵ����з�ʽ��ͬ��
2H2O+CH4������˵����������CO2�ĺ���Խ��Խ�á�����ͬ������˵���������ǣ���������ж�����̼�ĺ���̫�ͣ���Ӱ��ֲ��Ĺ�����ã�(5)���ʯ��ʯī���������ʲ���ϴ���Ҫԭ����̼ԭ�ӵ����з�ʽ��ͬ��

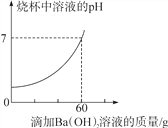
����Ŀ��ijƷ�ƽ����ijɷ�������������Ļ����Һ��ij����С����ⶨ��Ʒ�ƽ������Һ��HCl��������ȡ20g��Ʒ�ƵĽ������Һ���ձ��У����ϵμ�������������Ϊ17.1%������������Һ����Ӧ�������ձ��в��������������ձ�����ҺpHֵ�仯�IJ������������ͼ��ʾ��(��֪BaCl2��Һ��pH��7)
�μ�����������Һ������/g | 5 | 10 | 25 | 30 |
�ձ��в�������������/g | 1.165 | 2.33 | 4.66 | 4.66 |
��(1)��ȫ��Ӧ�����ɳ���������Ϊ________��
(2)����ý������Һ��HCl����������Ϊ___________��(������������0.1%)
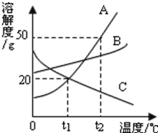
����Ŀ��KCl��KNO3���ܽ�ȱ����ܽ����������ͼ��ʾ������˵������ȷ���� ( )
�¶�/�� | 20 | 30 | 40 | 50 | |
�ܽ��S/g | KCl | 34.0 | 37.0 | 40.0 | 42.6 |
KNO3 | 31.6 | 45.8 | 63.9 | 85.5 | |
A. �ױ�ʾKCl�ܽ������ 
B. �¶�t1Ӧ��20����30��֮��
C. 40��ʱ��5gKCl����10gˮ�У��ɵõ�33.3%����Һ
D. 50��ʱ��30g KNO3����50gˮ������ܽ⣬�ٽ��µ�30�棬�о�������
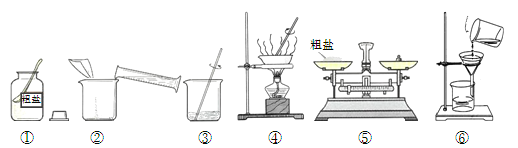
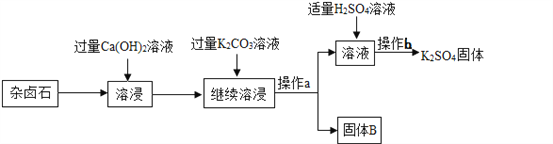
����Ŀ����±ʯ��K2SO4��MgSO4��2 CaSO4��2H2O�����ڡ�����Ϊ�ܳ�����ü���Դ���ñ��͵�Ca(OH)2��Һ����±ʯ�Ʊ�����أ������������£�
��֪��
���� | CaSO4 | K2SO4 | Mg��OH��2 |
�ܽ��/g(25��) | 0.28 | 11.1 | 0.00084 |
��1������a������Ϊ________���ò����в�������������___________��
��2������B����Ҫ�ɷ���________��________��
��3������b�IJ���������Ũ����________�����ˡ�ϴ�ӡ�
��4����ʵ�������м��������K2CO3��Һ���ܳ�ȥ��������______________��
��5����ʹ��Ca(OH) 2��Һ��K2CO3��Һ����±ʯ���С��ܽ���ʱ��Ϊ̽����ýϸ߽�����±ʯ��Ҫ�ɷֵĽ�ȡ�ʣ�ijʵ��С���ò�ý�ȡҺ��K+��������������ʾ��ȡ�ʣ�����ͬ��ʱ������������ʵ���飺
ʵ�� | �¶�/�� | Ca��OH��2����/g | K+��ȡ��/% |
�� | 25 | 2.5 | 72.4 |
�� | 40 | 2.5 | 86.55 |
�� | 40 | 3 | 90.02 |
�� | 60 | 3 | 91.98 |
�� | 60 | 4 | 91.45 |
������ʵ��������У��¶���K+��ȡ�ʵĹ�ϵ��_______________
��������г���5��ʵ���У���Ҫ�����ѵ�K+��ȡ�ʣ�ѡ��ķ�Ӧ������________����_____________g ��Ca(OH)2����