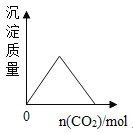
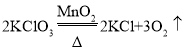��Ŀ����
����Ŀ����ͼ��ij����С�������ʵ������ȡ������װ��ͼ��������Ҫ�ش��������⡣
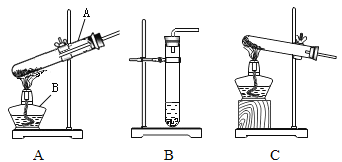
��1��д���������������ƣ�A_______
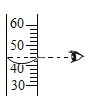
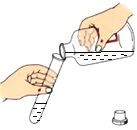
��2��ʵ�������ù���������Һ�Ͷ���������ȡ����Ӧѡ���װ��Ϊ______������ţ���ͬ�������{�������ȡ����ʱ��Ӧѡ���װ��Ϊ_____�����п�ѡ��Ĺ�ͬ������______��
�ٻ�ѧ�Լ� �ڴ����ӵ��Թ� �۴����е�����̨ �ܾƾ���
��3��ʵ�������ù���������Һ�Ͷ������̵Ļ������ȡ���������ֱ���ʽΪ_____�����ж������̵�����Ϊ_______��
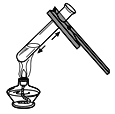
��4������װ������һ�������������Dz������ģ�װ���еIJ�����֮��Ϊ����_______����_______��
��5��ʵ�������{�������ȡ���ռ��ϴ�����������Ҫ��Ϊ���¼�������װҩƷ����˨��װ�������ԣ����ռ����壻�̶ܹ�װ�ã��ݼ��ȣ��ްѵ��ܴ�ˮ����ȡ������ֹͣ���ȡ�ʵ�����˳����ȷ����_______��
A �٢ڢۢܢݢޢ�
B �ۢ٢ڢݢܢޢ�
C �ڢ٢ܢݢۢޢ�
D �٢ܢڢۢݢߢ�
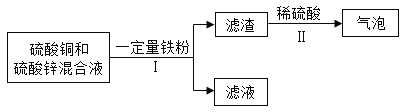
��6����ش���̽����У�������������⡢�����ϡ���������衢���ʵ�鷽�����⣬�����ܾ�������Щ��_______________��
���𰸡��Թ� B C �ڢ� ��������![]() ˮ+���� �����ã���ı䷴Ӧ���ʣ� �Թܿ������� �������������Թܹ��� C ����ʵ�顢�ռ�֤�ݡ���������ۡ���˼������
ˮ+���� �����ã���ı䷴Ӧ���ʣ� �Թܿ������� �������������Թܹ��� C ����ʵ�顢�ռ�֤�ݡ���������ۡ���˼������
��������
��1�����������ƣ�A�Թܣ�
��2��ʵ�������ù���������Һ�Ͷ���������ȡ������Ӧѡ���Һ����װ��B�����{�������ȡ����ʱ��Ӧѡ��������װ��C�����п�ѡ��Ĺ�ͬ������
�ٻ�ѧ�Լ���ͬ����˵������
�ڴ����ӵ��Թܣ���װҩƷ����˵����ȷ��
�۴����е�����̨���̶���������˵����ȷ��
�ܾƾ��ƣ�����������Һ�Ͷ���������ȡ��������Ҫ���ȣ���˵������
��ѡ���ڢۣ�
��3��ʵ�������ù���������Һ�Ͷ������̵Ļ������ȡ���������ֱ���ʽΪ����������![]() ˮ+���������ж������̵�����Ϊ�������ã�
ˮ+���������ж������̵�����Ϊ�������ã�
��4��Aװ�������������Dz������ģ�װ���еIJ�����֮��Ϊ�����Թܿ������ϣ��ڲ������������Թܹ�����
��5��ʵ�����˳����ȷ���ǣ����顢װ�������㡢�ա��롢Ϩ��
��ѡ��C �ڢ٢ܢݢۢޢ�
��6����ش���̽����У�������������⡢�����ϡ���������衢���ʵ�鷽�����⣬�����ܾ���������ʵ�顢�ռ�֤�ݡ��ܽ���ۡ���˼�����ۣ�

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ����ͼ��ʵ������ȡ����IJ���װ�ã���ش��������⣺
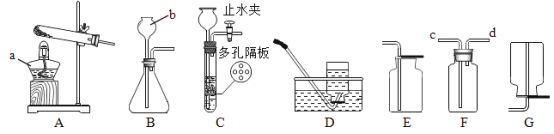
��1��д�����������ƣ�a ��_____��b ��_____��
��2��ѡ�� A װ���������Ļ�ѧ����ʽ_____���ռ��Ƚϸ����������ѡ����ռ�װ����_____����д��ţ���
��3��ʵ������ CO2 ��ѡ C ��Ϊ����װ�ã���װ�õ��ŵ���_____���ռ���һ�����������н�ֹˮ�У�C װ���й�����Һ�����ֿ������ܵ�ԭ����_____������ F װ���ռ� CO2������Ӧ�ô�_____������c������d������ͨ�룬�����ķ���Ϊ_____��
��4��ijͬѧ���Ե��ص�ʯ��ʯ��ʯ��Ʒ���м��飬ȡ�� 50.0g ����ʯ��ʯ��Ʒ���� 200.0g ϡ����� 4 �μ��룬 ʵ������������ݼ��±�����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ����
��� | ����ϡ����������g�� | ʣ�����������g�� |
�� 1 �� | 50.0 | 40.0 |
�� 2 �� | 50.0 | M |
�� 3 �� | 50.0 | 20.0 |
�� 4 �� | 50.0 | 12.0 |
����㣺
��. ���� m ����ֵӦ��Ϊ_____��
��. ʯ��ʯ��Ʒ��̼��Ƶ�������_____g��
��. �μӷ�Ӧ��ϡ�����������������_____?�����ݻ�ѧ����ʽ��ʽ���㣩����ȷ�� 0.1%��