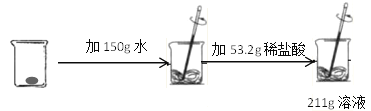
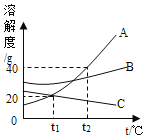
��Ŀ����
����Ŀ��С���ڽ�������һС��ũ�أ���ĩ��ʱ��ͼ���һ��ȥ�������Ȼ�ķ羰���滺����ѹ���Լ���������˵ĸ��顣�����������һЩ���⣬������������
(1)�������ֵ�����Ҷɫ���ƣ���������ѧ֪ʶ֪������ȱ�����ʵ�һ�ֱ��֡��������Ӧ��ʩ��____(����)����K2SO4��NH4NO3��Ca(OH)2��Ca(H2PO4)2
(2)�������˵�ϲ���ߣ��о���һ�ݿ�ѧ��Ӫ��ʳ�ף���ͼ��ʾ��

Ϊ�˴ﵽ��ѧ��ʳ�ı�������Ϊ����ʳ���л�ȱ��_______Ӫ���أ�����Ϊ����ʳ������һ�ָ�ʳ_______(���������)��
���𰸡� �� ά���� �ȡ������˵�
�����������⿼���˻��ʵķ���;���Ӫ���뽡����
��1����K2SO4�к��м�Ԫ�أ����ڼطʣ�����NH4NO3�к��е�Ԫ�أ����ڵ��ʣ���ȷ����Ca(OH)2�в�����Ԫ�أ����ǵ��ʣ���������Ca(H2PO4)2������Ԫ�أ������ʣ�����ѡ����
��2��������Ҫ������Ӫ�����ʣ������ʡ����ࡢ��֬��ά���ء����κ�ˮ�����и������ۣ������������࣬�����㡢�Ź��к�����֬�������ʺ����Σ�����������Ҫ���е����ʼ�ˮ�����Σ�����ʳ���л�ȱ��ά���أ�ΪʹӪ��������Ӻ�������Ҫ���主��ά���ص�ʳ��ȡ��������к��зḻ��ά���ء�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
��1�����������������������Һ�ֽ������Ƿ��й��أ���������¶Ա�ʵ�飺
��3.0g 10%H2O2��Һ��1.0g MnO2���Ȼ�ϣ�
��x g 10%H2O2��Һ��1.0g CuO���Ȼ�ϡ�
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
���з�Ӧ�Ļ�ѧ����ʽ��__________________________��
����x��ֵӦΪ_____________g��
����������������ʱȢ�죬�ɴ˵ó���ʵ�������______________________��
��2����̽����Ӱ�����������Һ�ֽ��ٶȵ�ij�����ء�ʵ�����ݼ�¼���£�
����������Һ������ | ����������Һ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
�� | 50.0g | 1% | 0.1g | 9mL |
�� | 50.0g | 2% | 0.1g | 16mL |
�� | 50.0g | 4% | 0.1g | 31mL |
��ʵ���У�����O2�����װ����______________�����ţ���
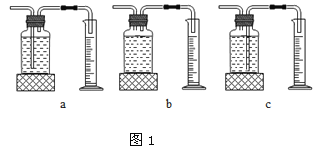
ʵ����ۣ�����ͬ�����£�___________________������������Һ�ֽ��Խ�졣
��3��������ͼ2װ�ý���ʵ�飬ͨ���Ƚ���ͬʱ����____________Ҳ�ܴﵽʵ��Ŀ�ġ�