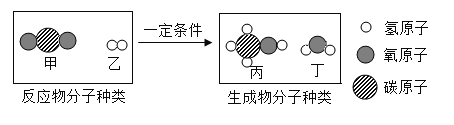
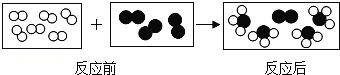
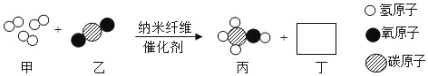
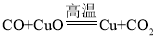��Ŀ����
����Ŀ��ij��ѧ������ʦָ����̽������ˮ�����ķ�Ӧ��
(1)��ͼ��װ��ҩƷ������װ��(�г���������ȥ)������Aװ�õ�������________��
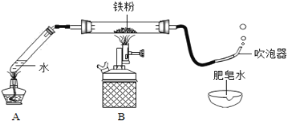
(2)����һ��ʱ���ɫ������ڣ������������������ݣ����������Ϸ�����ȼ�ŵ�ľ���������ݣ��ܲ���������������ȼ�յĻ�ѧ����ʽΪ_____________��
(3)ͬѧ�����ۺ���Ϊ������ˮ������Ӧ���ɵĹ�����������һ�������������������ڵĺ�ɫ�����л����ܺ�����������������
(��������)��������������(FeO��Fe2O3��Fe3O4)������FeO�Ӵ��������������ɺ�ɫ��Ϊ����ɫ���������������ﶼ��������ͭ��Һ��Ӧ��
(ʵ��̽��)
ʵ�鲽�� | ʵ������ | ʵ����� |
�����������к�ɫ���壬ƽ���ڰ�ֽ�� | ��ɫ���岻��ɫ | ��ɫ������һ��û��____(����������) |
ȡ������ɫ����������װ���Թܣ���������____��Һ | ________�����к�ɫ������� | ��ɫ������һ������__________ |
(̽������)����ˮ���������û���Ӧ���йصĻ�ѧ����ʽ��________________________��
���𰸡��ṩˮ���� 2H2+O2![]() 2H2O ���������������� ����ͭ ��ɫ���岿���ܽ� �������������� 3Fe+4H2O
2H2O ���������������� ����ͭ ��ɫ���岿���ܽ� �������������� 3Fe+4H2O![]() Fe3O4 +3H2
Fe3O4 +3H2
��������
��ʵ��Ϊ����ˮ������Ӧ��װ��A����ˮ��Ŀ����Ϊʵ���ṩˮ������
(2)��ȼ�ŵ�ľ���������ݣ��ܲ���������֤��������Ϊ��ȼ�����壬��Ϊ��Ӧ���к�����������������Ԫ�أ��������ɵĿ�ȼ�������������������������ڵ�ȼ�����·�Ӧ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��2H2+O2![]() 2H2O��
2H2O��
(3)������Ϊ����ɫ����������������Ӵ����ɺ�ɫ��Ϊ����ɫ������������������Ϊ��ɫ�������������к�ɫ���壬ƽ���ڰ�ֽ�ϣ���ɫ���岻��ɫ��˵����ɫ������һ��û����������������������ɫ������һ������������������������������������������ͭ��Һ��������ѧ��Ӧ��ȡ������ɫ����������װ���Թܣ�������������ͭ��Һ����ɫ����ӦΪ�����ܽ⣬���к�ɫ������֣�ͭ����Ϊ��ɫ��֤����ɫ�����к�������������������
[̽������]�ڸ��������£�����ˮ���������û���Ӧ������������������������Ӧ���е�ˮ����Ϊ���壬�������е�����Ϊ���岻�ü��ϼ�ͷ���仯ѧ����ʽΪ��3Fe+4H2O![]() Fe3O4+3H2
Fe3O4+3H2

����Ŀ��ij������ͬѧ����ϡ���������������Һ����������ʵ�飺
��1����һС��ͬѧ����9.8%��ϡ�����10%������������Һ�кͷ�Ӧ�������¶ȵı仯��
�ٵ�һ����������Һ�����ƺͱ���
���ȣ���С��ͬѧ�����ƺõ�10%������������Һ�������_____����ϸ��ƿ�У����á���Σ�����ͬѧ�������ǩ��ʾ��Ũ��������100g������������Ϊ9.8%��ϡ���ᡣ����ʽ��������ˮ�����_____��
����
��ѧʽH2SO4
��Է�������98
��������98%
���Լ�Ϊ��ɫ����ճ��Һ��
�и�ʴ��!
�ڸ�С��ͬѧ��ȡ��8���Ϊ8mL������������Һ���ֱ������м���һ�������ϡ���ᣬ����¶ȱ仯�����ʾ��
ʵ����� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
����ϡ�������� ��V��/mL | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 |
��Һ�¶����� ����t��/�� | 4.8 | 8.4 | 11.2 | 11.1 | 13.1 | 11.4 | 10.2 | 9.2 |
����ʵ�����Ϊ_____����ʵ��������ţ���һ���������Բ��������ж�������_____��
��2���ڶ�С��ͬѧ�ƻ�̽���кͷ�Ӧ�����е�pH�仯���������ȡϡ�ͺ������������Һ25g�������м���������������Ϊ9.8%��ϡ���ᣬ����ϡ�������������ҺpH�ı仯�����ͼ��ʾ�����㣺
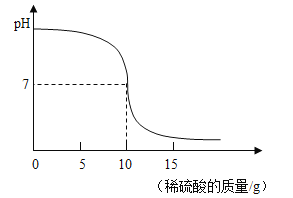
�ٵ�����ǡ����ȫ�к�ʱ��������Һ�����ʵ���������_____������������ȷ��0.1%��
����֪��20��ʱ�����Ƶ��ܽ��Ϊ19.5g����Ҫ����Ӧ��������Һǡ��ת��Ϊ20��ʱ�ı�����Һ����ķ�����_____��