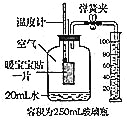
��Ŀ����
����Ŀ����Դ���������������������ɳ�����չ������أ�����Ӧ���� ����̼��������
��1��Ŀǰ����ʹ�õ�ȼ�ϴ�������ڻ�ʯȼ�ϣ���ú��ʯ�͡���Ȼ���ȡ����к���ú����ȫȼ��ʱ������������Ⱦ����______________��
��2�������ѣ�CH3OCH3������Ϊ�� 21��������ȼ�ϣ����ڿ����г��ȼ������ˮ�Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ��________________��
��3����������������Դ�ǽ����Դ�ͻ����������Ҫ;����������������Դ���� ______������ĸ��ţ���
A ����
B ̫����
C ������
D ʯ��
��4������дһ�����ܼ��ŵĽ��� _________________��
���𰸡�CO��SO2 CH3OCH3+3O2![]() 3H2O+2CO2 ABC �������У��ٿ�˽�ҳ����������ɣ�
3H2O+2CO2 ABC �������У��ٿ�˽�ҳ����������ɣ�
��������
��1������ú����ȫȼ��ʱ����һ����̼�Ͷ��������CO��SO2��
��2��������ȼ������ˮ�Ͷ�����̼����ѧ����ʽΪCH3OCH3+3O2![]() 3H2O+2CO2�����CH3OCH3+3O2
3H2O+2CO2�����CH3OCH3+3O2![]() 3H2O+2CO2��
3H2O+2CO2��
��3������Դ�������ܡ�̫���ܡ������ܣ�ʯ���ǻ�ʯȼ�ϣ�����������Դ����ѡ��ABC��
��4�����ܼ��ŵĽ��飺�������У��ٿ�˽�ҳ��ȣ��������ɣ���

����Ŀ���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ��ѧϰ���ص㡣�ס��ҡ���������ʾ�������ʣ����ǵ���ʾ��ͼ�����ʾ
���� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
|
��1��һ�������ӹ���_____��ԭ�ӡ�
��2���ס�������������һ�������·�Ӧ���ɱ��Ͷ�����Ӧ�Ļ�ѧ����ʽΪ_____�μӷ�Ӧ�ļ��ҵķ��Ӹ�����Ϊ_____��
����Ŀ������ij��ѧС���ͬѧ��ѧϰ���кͷ�Ӧ������ʵ���������ǽ�һ���� ������������Һ��ϡ�����ϣ���ͼ 1 ��ʾ���䷴Ӧ�Ļ�ѧ����ʽΪ_____�� ͬѧ�Ƿ��ָ÷�Ӧû�����Ե�������������ʵ��̽����
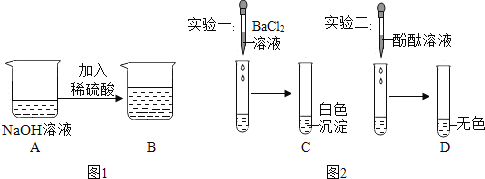
��������� 1�������֤�÷�Ӧ�Ѿ�������
������ʵ�飩ͬѧ�Ƿֱ�ȡͼ 1B ͼ����Һ������������ʵ�飬�����ͼ 2 ��ʾ��
����Ӧ���ۣ�����Ϊ����ͼ 2 ��ʵ������֤��Ӧ�Ѿ���������ʵ�� _____����ѡ�� ������һʵ����۽Ƕȷ�������ȷ����������_________��
��������� 2��ͼ 1B ͼ��Һ�����ʵijɷ�����Щ��
������ʵ�飩
ʵ����� | ʵ������ | ���� |
ȡ B ͼ�е�������Һ���Թ� �м���_____�� | �۲쵽_____�� | B ͼ����Һ��������Na2SO4 �� H2SO4 |
��˼����ͬѧ����Ϊ����һ�ֲ�ͬ�����Լ� _____��Ҳ�ܴﵽ�� ͬ��Ŀ�ġ�
����չ���죩�� C ͼ�Թ��е����ʾ��ã��ϲ���Һ��һ�����е�������_____��