��Ŀ����
ij��ѧ��ȤС���ͬѧ��һƿ���õ���ʯ�ҷ�ĩ����ɽ���ʵ��̽��������һ��������ǵ�̽�����
[�������]��ƿ��ʯ�ҷ�ĩ�Ƿ��Ѿ�����������CaCO3��
[���в���]����һ����ʯ��ȫ�������CaCO3������ ���������ʯ�Ҳ��ֱ����CaCO3��
����������ʯ��û�б��ʣ�
[���ʵ��]��С��ͬѧ�Բ���һ���������̽�����������������������±������ʵ�����ݣ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ����������ˮ�����裬���� ��ȡ������Һ���Թ��У������̪��Һ ��ȡ�����������Թ��У��������� | ��______�� ��______�� | ����һ���� |
��2��С����ⶨ��ʯ����Ʒ��Ca��OH��2��������������ȡ����1.0g��ʯ����Ʒ��������ˮʹ֮����ܽ⣬������˺���ʵ������pH��ֽ�����ƺõ�10%������Һ����ʵ�飬ʵ�����ݼ�¼���±���
| ����������Һ������/g | 0 | 4 | 7.3 | 12 |
| ��Ʒ��pH | 12 | 9 | 7 | 4 |
���������ʯ����Ʒ��Ca��OH��2������������
�⣺[���ʵ��]������һ������˵����Ʒ��û��Ca��OH��2������Һ�����ԣ������̪��Һ�����������������м���ϡ�������CaCO3��ϡ���ᷴӦ������̼��ƺ�ϡ���ᷴӦ������CO2�����Ի������ݲ�����
�ʴ�Ϊ���ڡ����������ۡ������������ݣ�����ȫ���ܽ⣮
[��˼��Ӧ��]���������������˵����Ʒ�л��в���Ca��OH��2������Һ�ʼ��ԣ��ڼ����̪��Һ���ʹ��̪��죮
�ʴ�Ϊ����Һ��죮
��2�����١���PH=4ʱ��˵����Һ�����ԣ�����Ŀ�п��Կ���ԭ��һ���Ǽ���������������Ӧ�����ɵ���Һ�����������ʳ������ɵ�CaCl2�⣬����ʣ�������е�����HCl��
�ʴ�Ϊ��CaCl2��HCl��
��2�����ڡ��������֪���������������Ϊ7.3gʱ�������Ca��OH��2ǡ����ȫ��Ӧ��
��μӷ�Ӧ��HCl������Ϊ7.3g��10%=0.73g
�裺��Ʒ��Ca��OH��2������Ϊx
Ca��OH��2+2HCl=CaCl2+2H2O
74 73
x 0.73g
 =
=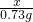
x=0.74g
��Ʒ��Ca��OH��2����������Ϊ��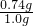 ��100%=74%
��100%=74%
����Ʒ��Ca��OH��2����������Ϊ74%��
��������ʯ�ұ�����ָCa��OH��2�Ϳ����еĶ�����̼��Ӧ����CaCO3��
��[���ʵ��]�У����Ľ����Dz���һ������������ʯ��ȫ�������CaCO3�����Զ϶���Ʒ��û��Ca��OH��2������Һ�����ԣ���̪����ɫ������ȫ��ΪCaCO3���������ȫ���ܽ⣬������������̼��
��[��˼��Ӧ��]�У��������������˵����Ʒ�л��в��ֵ�Ca��OH��2������Һ�ʼ��ԣ���̪��죮
��2������PH=4��˵����Һ�����ԣ���������Һ����������Һ�����������������֣��ֱ��Ƿ�Ӧ���ɵ�CaCl2��ʣ��� HCl��
�ڡ�Ҫ������Ʒ��Ca��OH��2���������������Ը��ݵ���ҺΪ����ʱ���ĵ�������Һ��������������Ʒ��Ca��OH��2����������Ϊ��Һ������˵�������Ca��OH��2ǡ����ȫ��Ӧ��
����������һ���ۺ��Ժ�ǿ��ʵ����ͼ����⣮ʵ�鲿��Ҫ���ݽ����Ƴ�ʵ��������Ҫע��һЩ�ؼ��Ĵʣ������һ�еġ�ȫ������������еġ����֡������㲿�ֹؼ���Ҫ������������ʾ������--��Һ�����Ծͱ�ʾǡ����ȫ��Ӧ��
�ʴ�Ϊ���ڡ����������ۡ������������ݣ�����ȫ���ܽ⣮
[��˼��Ӧ��]���������������˵����Ʒ�л��в���Ca��OH��2������Һ�ʼ��ԣ��ڼ����̪��Һ���ʹ��̪��죮
�ʴ�Ϊ����Һ��죮
��2�����١���PH=4ʱ��˵����Һ�����ԣ�����Ŀ�п��Կ���ԭ��һ���Ǽ���������������Ӧ�����ɵ���Һ�����������ʳ������ɵ�CaCl2�⣬����ʣ�������е�����HCl��
�ʴ�Ϊ��CaCl2��HCl��
��2�����ڡ��������֪���������������Ϊ7.3gʱ�������Ca��OH��2ǡ����ȫ��Ӧ��
��μӷ�Ӧ��HCl������Ϊ7.3g��10%=0.73g
�裺��Ʒ��Ca��OH��2������Ϊx
Ca��OH��2+2HCl=CaCl2+2H2O
74 73
x 0.73g
 =
=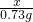
x=0.74g
��Ʒ��Ca��OH��2����������Ϊ��
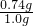 ��100%=74%
��100%=74%����Ʒ��Ca��OH��2����������Ϊ74%��
��������ʯ�ұ�����ָCa��OH��2�Ϳ����еĶ�����̼��Ӧ����CaCO3��
��[���ʵ��]�У����Ľ����Dz���һ������������ʯ��ȫ�������CaCO3�����Զ϶���Ʒ��û��Ca��OH��2������Һ�����ԣ���̪����ɫ������ȫ��ΪCaCO3���������ȫ���ܽ⣬������������̼��
��[��˼��Ӧ��]�У��������������˵����Ʒ�л��в��ֵ�Ca��OH��2������Һ�ʼ��ԣ���̪��죮
��2������PH=4��˵����Һ�����ԣ���������Һ����������Һ�����������������֣��ֱ��Ƿ�Ӧ���ɵ�CaCl2��ʣ��� HCl��
�ڡ�Ҫ������Ʒ��Ca��OH��2���������������Ը��ݵ���ҺΪ����ʱ���ĵ�������Һ��������������Ʒ��Ca��OH��2����������Ϊ��Һ������˵�������Ca��OH��2ǡ����ȫ��Ӧ��
����������һ���ۺ��Ժ�ǿ��ʵ����ͼ����⣮ʵ�鲿��Ҫ���ݽ����Ƴ�ʵ��������Ҫע��һЩ�ؼ��Ĵʣ������һ�еġ�ȫ������������еġ����֡������㲿�ֹؼ���Ҫ������������ʾ������--��Һ�����Ծͱ�ʾǡ����ȫ��Ӧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
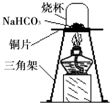 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����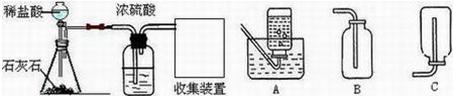
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������