��Ŀ����
����Ŀ�����꼶�������ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ���������ͼ��ʾ��
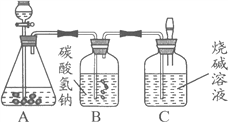
��������ϡ������뵽20 g����ʯ�У������ɷֲ������ᷴӦ�����Ѳ�����CO2�������������ռ���Һ���գ�ͬʱ����Cƿ�ռ���Һ���ӵ�������������±���ʾ��
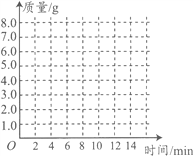
ʱ�� / �� | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
C��������/C | 0 | 3.0 | 5.0 | 6.0 | 6.6 | x | 6.6 |
(1)�ϱ��У���10����ʱ��x= ��
(2)�������ʯ��Ʒ��̼��Ƶ�����������
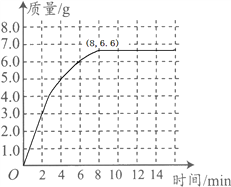
(3)������ͼ������ֽ�ϣ���ʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ���ߣ�
(4)B����װҩƷΪ̼��������Һ�����������տ��ܻӷ���HCl���壬����Ϊ�Բ��������û��Ӱ�죿 ���������û������
���𰸡�(1)6.6
(2)75%
(3) 
(4)��
����������1���ɱ���֪��������̼�����ﵽ6.6gʱ�����������ӣ�Ҳ���Ƕ�����̼���������6.6g�����ԣ���10����ʱ��x=6.6g��
��2������Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 6.6g
![]()
��ã�x=15g
����ʯ��Ʒ��CaCO3����������Ϊ![]() ��100%=75%��
��100%=75%��
��3���ɱ��е����ݿ�֪�����ɵĶ�����̼��ʱ��Ĺ�ϵ�������£�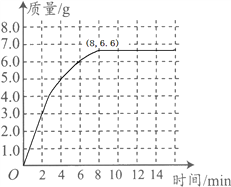 ��
��
��4������̼�������������ᷴӦ�����˶�����̼���壬����B����װҩƷΪ̼��������Һ�����������տ��ܻӷ���HC1���壬�Բ��������Ӱ�졣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�