��Ŀ����
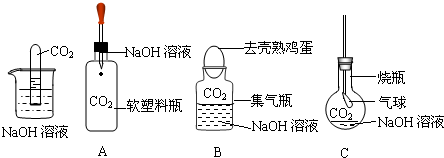
 ��ѧ��ȤС��ͬѧ�ڰ�����ʦ����ʵ����ʱ�������˰�ƿ���õ��������Ʒ�ĩ�����Ǿ��������ƿ�������Ʒ�ĩ����ɽ���ʵ��̽����
��ѧ��ȤС��ͬѧ�ڰ�����ʦ����ʵ����ʱ�������˰�ƿ���õ��������Ʒ�ĩ�����Ǿ��������ƿ�������Ʒ�ĩ����ɽ���ʵ��̽������1��������⣺��ƿ���������Ƿ��Ѿ����ʣ�
��2����������裺
���������Ʒ�ĩ�ijɷ�Ϊ���������ƣ����������ơ�̼��ƣ���̼���
��2����Ʒ���������ʵ�飺�������̽��������д�±���
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ��Ʒ������С�ձ��У����� ����ˮ����ֽ��裬���� | / | / |
| ��ȡ�����������Թ��У� �μ����� | �������ݲ��� | ����ڳ��� |
| ��ȡ������Һ���Թ��У� |
���Ӧ���ܷⱣ�森��д��һ��ҩƷ�����Ʋ�˵����ҩƷ��Ҫ�ܷⱣ���ԭ��
��5��ͨ��ʵ��̽����С��ͬѧ���������һЩ���飬���к�������
A���㵹Һ��ʱӦע�Ᵽ����ǩ������ʴ
B��Ҫ���Ͻ��Ŀ�ѧʵ���̬��
C�������ϵ�ҩƷ��ֱ�ӵ��������豣����
��6��ijͬѧΪ�ⶨ������ɫ��ĩ��̼��Ƶ�������ȡ��Ʒ17.4�ˣ�����87g�����У�����ȫ���ܽ⣬ǡ����ȫ��Ӧ������ձ�����Һ������Ϊ100�ˣ���ԭ��ĩ��̼��Ƶ����������Ƕ��٣�
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,��ʯ�ҵ���������;,��Ļ�ѧ����,�εĻ�ѧ����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
��������3����Ʒ���������ʵ�飺�������Ʊ��ʺ�����̼��ƣ�̼���������ˮ����������������ˮ���������ǿ��Ը����Ƿ�����ˮ�����ƶ��Ƿ���ʣ�����̼����������ᷴӦ���ɶ�����̼���壬Ȼ������Ƿ�������������ж��Ƿ��������̼��ƣ��������������������̼�ܷ�Ӧ���ɳ����ж���ĩ���Ƿ���δ���ʵ��������ƣ�
��4����ʾ���������Ʊ���������Ca��OH��2������еĶ�����̼������ѧ��Ӧ��Ե�ʣ������������Ӧ�ܷⱣ�森����Ũ����ȵı��森
��5�����ݽ�ԼҩƷ��ԭ�����۶��������ϵ�ҩƷ�Ĵ����������н��
��6��ʯ��ʯ��������ϡ�����������ȥ���ձ���ʣ�����ʵ��������õ����������ɶ�����̼���������ɶ�����̼������������̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�����Լ����ʯ��ʯ��̼��Ƶ�������������������
��4����ʾ���������Ʊ���������Ca��OH��2������еĶ�����̼������ѧ��Ӧ��Ե�ʣ������������Ӧ�ܷⱣ�森����Ũ����ȵı��森
��5�����ݽ�ԼҩƷ��ԭ�����۶��������ϵ�ҩƷ�Ĵ����������н��
��6��ʯ��ʯ��������ϡ�����������ȥ���ձ���ʣ�����ʵ��������õ����������ɶ�����̼���������ɶ�����̼������������̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�����Լ����ʯ��ʯ��̼��Ƶ�������������������
����⣺
��3����Ʒ���������ʵ�飺�������Ʊ��ʺ�����̼��ƣ�̼���������ˮ����������������ˮ���������ǿ��Ը����Ƿ�����ˮ�����ƶ��Ƿ���ʣ�����̼����������ᷴӦ���ɶ�����̼���壬Ȼ������Ƿ�������������ж��Ƿ��������̼��ƣ��������������������̼�ܷ�Ӧ���ɳ����ж���ĩ���Ƿ���δ���ʵ��������ƣ�
��4����ʾ���������Ʊ���������Ca��OH��2������еĶ�����̼������ѧ��Ӧ��Ե�ʣ������������Ӧ�ܷⱣ�森��Ϊ�ӷ�������������С����Ũ����ҲӦ�ܷⱣ�森
��5���㵹Һ��ʱӦע�Ᵽ����ǩ������ʴ����A������Ҫ���Ͻ��Ŀ�ѧʵ���̬�ȣ���B���������ڱ�ǩ����ʴ���Լ������Ž�Լԭ��Ӧ������ȷ��ҩƷ����ɣ����������������Ȳ���ȫ������˷ѣ���C����������ѡAB��
��6�����ɶ�����̼������Ϊ87g+17.4g-100g=4.4g
��ʯ��ʯ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
��
=
�����x=10g����ʯ��ʯ��̼��Ƶ���������Ϊ
��100%��57.5%
�ʴ�Ϊ����3��
��4��CO2+Ca��OH��2=CaCO3��+H2O��Ũ���ᣬ��Ϊ�ӷ�������������С����5��AB����6��57.5%
��3����Ʒ���������ʵ�飺�������Ʊ��ʺ�����̼��ƣ�̼���������ˮ����������������ˮ���������ǿ��Ը����Ƿ�����ˮ�����ƶ��Ƿ���ʣ�����̼����������ᷴӦ���ɶ�����̼���壬Ȼ������Ƿ�������������ж��Ƿ��������̼��ƣ��������������������̼�ܷ�Ӧ���ɳ����ж���ĩ���Ƿ���δ���ʵ��������ƣ�
��4����ʾ���������Ʊ���������Ca��OH��2������еĶ�����̼������ѧ��Ӧ��Ե�ʣ������������Ӧ�ܷⱣ�森��Ϊ�ӷ�������������С����Ũ����ҲӦ�ܷⱣ�森
��5���㵹Һ��ʱӦע�Ᵽ����ǩ������ʴ����A������Ҫ���Ͻ��Ŀ�ѧʵ���̬�ȣ���B���������ڱ�ǩ����ʴ���Լ������Ž�Լԭ��Ӧ������ȷ��ҩƷ����ɣ����������������Ȳ���ȫ������˷ѣ���C����������ѡAB��
��6�����ɶ�����̼������Ϊ87g+17.4g-100g=4.4g
��ʯ��ʯ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
��
| 100 |
| x |
| 44 |
| 4.4g |
| 10g |
| 17.4g |
�ʴ�Ϊ����3��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| / | / | |
| ��ϡ���� | ����ڳ��� | |
| �۵����̪��Һ | ��Һ���ɫ |
�����������������������̼��Ӧ����̼��ƣ���������������ˮ����̼���������ˮ����̼����������ᣮ��������������̼������ʵIJ�ͬ�����ʵ������������Ƿ�����DZ����һ����Ҫ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
6��5���ǡ����绷�������ա���2009���ҹ��������ں��ǡ�������Ⱦ���ж���������ij��ѧ�о�С����������������룬����Ϊ���к������е��ǣ�������
| A����ȼú�м����������Լ��١����ꡱ���γ� |
| B����������ˮ�ܡ�̫���ܵ������Դ����ֹʹ�û�ʯȼ�� |
| C���������Ͽɲ��÷��յķ����������Խ������ɫ��Ⱦ�� |
| D���ϸɵ��ʵʩ�������Է�ֹ��ˮԴ����������Ⱦ |
��ѧ�仯���������仯��ijͬѧ��̼���ơ�̼�����������ᷴӦ�����еķš������������̽��������Ϊ�������Լ�1�м����Լ�2�����衢���£����ټ����Լ�3�����衢���£���¼�������£�
������˵���У�����ȷ���ǣ�������
| �Լ�1 | �Լ�2 �¶�/�� | �ܽ���¶�/�� | ���ú��¶�/�� | �Լ�3 �¶�/�� | ��Ϻ��¶�/�� |
| 0.5gNa2CO3 | 10mL H2O 20.0 | 23.3 | 20.0 | 10mL HCl��20%�� 20.0 | 23.7 |
| 0.5gNaHCO3 | 10mL H2O 20.0 | 18.5 | 20.0 | 10mL HCl��20%�� 20.0 | 20.8 |
| A����������ˮ�Ĺ���Ҳ�����ȡ��������� |
| B��������ڣ�ͬ�����ԴﵽĿ�� |
| C��̼���������ᷴӦ�ų�������һ����̼�����Ʒ�Ӧ�ų��Ķ� |
| D������ʵ�����������ỻ����ͬŨ�ȵ�ϡ�����ʵ�����û��Ӱ�� |
���Ϲ�����2011��Ϊ�����ʻ�ѧ�ꡱ���Լ��ѧ��ȡ�õijɾ��Լ������������Ĺ���Ϊ��ּ�������ᷨ����������ּ���ǣ�������
| A���ƹ�ʳ�ò����κλ�ѧ���ʵ���ɫʳƷ |
| B����������������������ѧϰ��ѧ����Ȥ |
| C���ռ���ѧ֪ʶ��������ѧ���ף���߹��ڿ�ѧ���� |
| D����ѧ��һ�Ŵ��������ʵĿ�ѧ������Ȼ��ѧ�д������������Ŀ�ѧ���� |