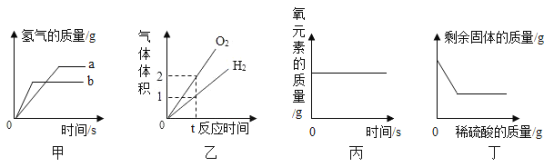
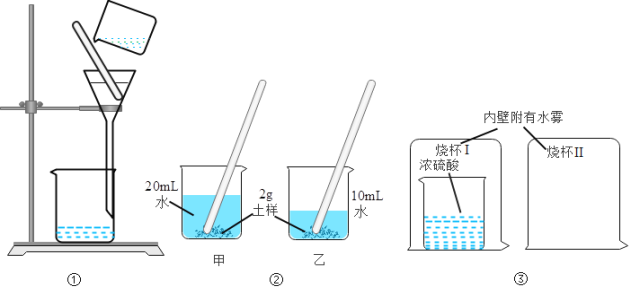
��Ŀ����
����Ŀ����ѧ�С���ͬѧ�Ժ��ǽ���ұ�����Ĺܵ�����Ĺ���ɷֽ��л��յ�̽������֪���ǽ���ұ�����Ĺܵ������к���̿�ڡ�����ͭ��п��
[�������]��δӹܵ������л��ս���ͭ��?
[ʵ�鷽�����]��ͼ��

[����ʵ��]
(1)ȡһ�����Ĺܵ�������Ʒ������������ϡ���ᣬ��ַ�Ӧ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______________��
(2)Ȼ����в���I_____________ (д��������) ���õ�����B�ijɷ�Ϊ______________��
(3)������B����ͭƬ�ϳ�����գ�Ŀ����______________��
(4)�����պ�Ĺ���ת�Ƶ��ձ��м���������ϡ���ᣬD�з�����Ӧ�Ļ�ѧ����ʽΪ______________��
(5)��D�õ�����Һ��������������۳�ֽ��貢���й���,�õ�������ͭ��������ͭ�к��е�������______________��
[���ɷ�˼]
(6)������ƺ�ʵ���еõ����Dz�����ͭ�����õ�����ͭ�����һ��ʵ�顣��ȥ����ͭ�е����ʣ���ѡ��_____________,�����)��
�ٴ���
��ϡ����
��Zn
���𰸡�![]() ���� ̿�ںͽ���ͭ�Ļ���� ��ȥ���������е�̿�ڣ�ͬʱ��ͭת��������ͭ
���� ̿�ںͽ���ͭ�Ļ���� ��ȥ���������е�̿�ڣ�ͬʱ��ͭת��������ͭ ![]() ���� �٢�
���� �٢�
��������
��1��A��п��ϡ���ᷴӦ��������п��������������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2����
��2��Ȼ����в���1�ǹ��ˣ��õ�����B�ijɷ���̿�ںͽ���ͭ�Ļ���
��3��������B����ͭƬ�ϳ�����գ�Ŀ���dz�ȥ���������е�̿�ڣ�ͬʱ��ͭת��������ͭ��
��4��D������ͭ��ϡ���ᷴӦ��������ͭ��ˮ��������Ӧ�Ļ�ѧ����ʽΪ��CuO+H2SO4=CuSO4+H2O��
��5����D�õ�����Һ��������������۳�ֽ��貢���й��ˣ��õ�������ͭ��������ͭ�к��е����������ۡ�
��6�����ܱ�����������ͭ���ܱ��������������������ᷴӦ��ͭ������ϡ���ᷴӦ��п������ͭ�����ܷ�Ӧ����ȥ����ͭ�е����ʣ���ѡ�ô�����ϡ���ᡣ
�ʴ�Ϊ��Zn+H2SO4�TZnSO4+H2�������ˣ�̿�ںͽ���ͭ�Ļ�����ȥ���������е�̿�ڣ�ͬʱ��ͭת��������ͭ��CuO+H2SO4=CuSO4+H2O�����ۣ��٢ڡ�